Many chemical process industries (CPI) facilities utilize high-pressure steam generators to produce both electricity and process steam. This technique is commonly known as combined heat and power (CHP). A popular approach in recent years has been to select a combined-cycle power and steam-generation unit to utilize the combination of a combustion turbine and a heat-recovery steam generator (HRSG) for process-steam generation and perhaps additional power generation. Owners and operators of these combined-cycle units, as well as other steam generators, need to be aware of the phenomenon known as flow-accelerated corrosion (FAC), which, if ignored, can lead to corrosion-induced failures of steam systems.
Dissolved oxygen treatment
Three decades ago, at the beginning of the author’s career in the utility industry, conventional wisdom said that any dissolved oxygen that entered the condensate and feedwater system of a high-pressure steam generator was harmful. At that time, over 50% of the power produced in the U.S. came from coal. Coal-fired steam-generation units typically have complex condensate/feedwater networks with numerous feedwater heaters. The prevalent thinking at the time was that any trace of dissolved oxygen (DO) would cause corrosion. And indeed, dissolved oxygen can be very problematic in uncontrolled environments. Therefore, virtually all feedwater systems for high-pressure steam generators were equipped with a deaerator for dissolved gas removal. A properly operating deaerator can lower DO concentrations to 7 parts per billion (ppb).
 |
 |
However, even this residual DO concentration was still considered harmful, so chemical deaeration was also the convention at most plants. The workhorse for chemical deaeration was hydrazine (N2H4), a reducing agent that reacts with oxygen according to the following reaction:
N2H4 + O2 → 2H2O + N2(g) (1)
Hydrazine proved advantageous because it does not add any dissolved solids to the feedwater, it reacts with oxygen in a one-to-one weight ratio, and it is supplied in liquid form in solutions with concentrations of 35%. Also, a primary benefit of hydrazine is that it will passivate oxidized areas of piping and tube materials as follows:
N2H4 + 6Fe2O3 → 4Fe3O4 + N2 (g) + 2H2O (2)
N2H4 + 4CuO → 2Cu2O + N2(g) + 2H2O (3)
Hydrazine residuals were typically maintained at relatively low levels of perhaps 20 to 100 ppb. Oxygen scavenger treatment was coupled with a feed of ammonia or an amine to maintain feedwater pH within a mildly alkaline range — 8.8 to 9.1 for mixed-metallurgy feedwater systems and slightly higher for all-ferrous systems.
NH3 + H2O → NH4 + + OH– (4)
This program became known as all volatile treatment [AVT(R)].
Due to the suspected carcinogenic nature of hydrazine, alternative chemicals, such as carbohydrazide, methyl ethyl ketoxime and others gained popularity, but the intent remained the same — to establish a reducing environment in the feedwater circuit, thus inhibiting oxidation of metal. The AVT technique became a standard in the industry.
A condensate-system failure on Dec. 9, 1986 changed the situation. A pipe elbow in the condensate system of the Surry Nuclear Power Station (near Rushmere, Va.) ruptured, and the failure caused four fatalities and tens of millions of dollars in repair costs and lost revenues [1]. As a result of this accident and others similar to it, researchers learned that the reducing environment produced by the oxygen-scavenger feed results in single-phase FAC.
The corrosive attack occurs at flow disturbances, such as elbows in feedwater piping, and economizers, feedwater-heater drains, locations downstream of valves and reducing fittings, attemperator piping, and, most notably for the combined-cycle industry, in low-pressure evaporators.
Single-phase FAC
The chemistry of single-phase FAC and its potentially catastrophic effects are outlined here. With single-phase FAC, wall-thinning occurs gradually until the remaining material at the affected location can no longer withstand the process pressure, whereupon catastrophic failure occurs.
When a steam generator is placed into service, carbon-steel feedwater piping and waterwall tubes form a layer of protective iron oxide known as magnetite (Fe3O4). Magnetite is actually a composition of FeO (with iron in a +2 oxidation state) and Fe2O3 (with iron in a +3 oxidation state).
The combination of a reducing environment (caused by the oxygen scavenging species) and localized fluid-flow disturbances causes the dissolution of ferrous ions (Fe+2) from the metal and from the metal-oxide matrix. Well known data from the Electric Power Research Institute (EPRI; Palo Alto, Calif.; www.epri.com) show that iron dissolution is greatly influenced by not only reducing conditions, but also by solution pH and temperature.
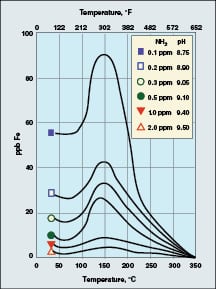 |
|
FIGURE 3. The dissolution of the carbon-steel matrix
is a function of pH and temperature [1] |
As Figure 3 illustrates, corrosion reaches a maximum at 300oF. Thus, feedwater systems and HRSG low-pressure evaporators are particularly susceptible locations. Also note the influence of pH — as reflected by ammonia concentration — on the corrosion characteristics. This factor becomes quite important with regard to controlling FAC.
In large measure, coal-plant personnel have recognized the problem of single-phase FAC, and have adopted alternative feedwater-treatment methods to mitigate the issue. Elsewhere however, such as in CHP projects within the CPI, proposals for combined-cycle steam and power units often specify an oxygen-scavenger feed system.
Solutions to single-phase FAC
HRSGs, by their nature, typically have many waterwall tubes with short-radius elbows. Thus, the HRSG unit contains many locations where single-phase FAC could be a concern. A primary method to mitigate this attack is to select the proper feedwater treatment scheme.
Approximately 40 years ago, researchers and chemists in Germany and Russia began using a program known as oxygenated treatment (OT) to minimize carbon-steel corrosion and iron dissolution in supercritical steam generators. The key component of the program was, and still is, deliberate injection of pure oxygen into the condensate/feedwater network to establish oxygen residuals of up to 300 ppb. What chemists discovered is that in very pure feedwater (cation conductivity ≤ 0.15 µS/cm (microsiemens per cm), the oxygen will intersperse and overlay magnetite to generate a tenacious and very insoluble film of ferric oxide hydrate (FeOOH). The OT approach typically lowered feedwater iron concentrations to 1 ppb or less, and, as researchers have subsequently confirmed, greatly minimized single-phase FAC. Currently, OT is the preferred feedwater treatment for most once-through utility steam generators around the world. In the U.S., an oxygen residual range of 30 to 150 ppb is common, with a recommended pH range of between 8.0 and 8.5. OT has been applied to a few drum units, where EPRI guidelines call for a feedwater pH range of 9.0 to 9.4, with a dissolved oxygen concentration of 30 to 50 ppb.[1]
Although OT has been successfully applied to drum boilers, another program has evolved that is very popular for condensate/feedwater in these steam generators. It is known as all-volatile treatment, oxygen [AVT(O)]. With AVT(O), oxygen is not deliberately injected into the condensate, but rather the amount that enters from condenser air in-leakage, according to “normal” conditions (we will examine “normal” conditions shortly) is allowed to remain without any oxygen-scavenger or metal-passivator treatment. It should be noted at this point that neither OT nor AVT(O) are permissible for feedwater systems containing copper alloys, as the oxygen would simply be too corrosive to the metal. The following text therefore focuses upon AVT(O) for all-ferrous systems.
When researchers developed AVT(O), they took into account the pH effect on carbon-steel dissolution, as previously illustrated in Figure 3. AVT(O) guidelines evolved to the following parameters:
Recommended pH range: 9.2–9.6
Feedwater dissolved oxygen concentration: ≤10 ppb
As with OT, the condensate must be quite pure to allow oxygen to generate the FeOOH protective layer rather than cause pitting. However, the cation-conductivity upper limit with AVT(O) is a bit more relaxed at ≤ 0.2 µS/cm.
A relatively new twist has emerged regarding AVT(O) philosophy. Chemists have discovered that the heretofore established limit of 10 ppb DO in the feedwater may allow single-phase FAC at some isolated locations in feedwater systems where eddy effects appear to prevent the dissolved oxygen from reaching the metal surface. It is the author’s understanding that guidelines may be modified to raise the upper DO limit to 20 ppb.
The amount of air in-leakage that establishes the “normal” condition of 10-ppb dissolved oxygen in the condensate is not a hard and fast value. The old rule of thumb for proper condenser conditions is a limit of 1 scfm (standard cubic feet per minute) of air in-leakage per 100 MW of capacity.
 |
|
FIGURE 4. The effects of two-phase FAC are
shown here in a deaerator |
However, the author has worked with units in which the air in-leakage ratio was significantly higher, but where the condenser vacuum pumps had sufficient capacity to remove the gases. Quite often, a failure at the condenser shell or within auxiliary equipment may cause a sudden spike in dissolved oxygen concentration. As contrasted to pure oxygen feed, such as with OT, air in-leakage also allows carbon dioxide to be drawn into the condensate, which raises the conductivity. In such cases, plant personnel need to search for the leak or leaks and repair them promptly. Much more problematic is a condenser tube leak, which not only raises the condensate dissolved solids concentration, but introduces impurities to the steam generator. These effects can be quite dramatic [2].
Chemical and material select
Elevated pH also has a beneficial effect in mitigating FAC. Thus, the guidelines for feedwater pH now recommend a range of 9.2 to 9.6. With EPRI’s phosphate continuum program or with caustic treatment alone, the drum pH can be controlled within a range of 9 to 10 quite readily.
A complication sometimes arises with HRSGs. Most HRSGs are of the multipressure, drum, vertical tube style. In some cases, the feedwater circuit is designed such that the feedwater enters each pressure circuit separately. In many others, however, the entire feedwater stream is routed to the low-pressure (LP) evaporator for additional heating before being distributed to the intermediate-pressure (IP) and high-pressure (HP) steam generators. In these situations, phosphate or caustic feed to the LP circuit is not permissible due to the downstream effects on attemperator (steam-temperature controller) chemistry, and IP and HP economizers. For these situations, the LP pH must be controlled via ammonia or amine injected into the feedwater. If the condenser uses tubes made from ferrous materials, the pH may be taken higher than the 9.2 to 9.6 range listed above without ill effects. However, copper-alloy tubes would suffer from extensive corrosion at the higher concentrations of ammonia.
For new HRSGs, single-phase FAC control can also be addressed in large measure by materials selection. The addition of a small amount of chromium to FAC-susceptible spots virtually eliminates the corrosion. A primary example is LP waterwall elbows. Fabrication of the elbows from 1¼ or 2¼ chrome alloy can provide great benefit. While this alloy addition adds some cost to the project, the materials are quite resistant to FAC.
Two-phase FAC
Many steam generators, regardless of type, are susceptible to two-phase FAC. As the name implies, this corrosion mechanism occurs where water flashes to steam, resulting in a mixed-phase fluid.
For conventional units, feedwater heater shells and heater drains are common locations for two-phase FAC, but this equipment is not common for HRSGs. However, de-aerators also experience two-phase fluid flow. As fluid flashes upon entering a de-aerator, oxygen departs with the steam. Thus, the water that impinges upon metal surfaces does not maintain an oxidizing environment. Also, the pH of entrained water droplets within the steam is usually lower than the bulk water pH. The combination of these factors often initiates FAC.
As has been noted, elevated pH will help to mitigate FAC, but the HRSG configuration dictates the maximum treatment allowed. If the LP system is utilized for heating of feedwater to the IP and HP circuits, solid alkali treatment (trisodium phosphate or caustic) of the LP circuit is not permissible. Control of pH can only be accomplished by ammonia, but it should be noted that ammonia hydrolysis, as previously outlined in Equation 4, decreases with increasing temperature.
As with single-phase FAC, a method to combat two-phase FAC is fabrication of susceptible locations with chromium-containing steel.
This article outlines the primary issues behind the recommended non-use of oxygen scavengers in all-ferrous, high-pressure steam generators. Certainly for lower-pressure industrial and auxiliary boilers, treatment programs may be more flexible. Methods and programs to detect FAC in existing plants can be found in [1].
Edited by Scott Jenkins
References
1. Cycle Chemistry Guidelines for CombinedCycle/Heat Recovery Steam Generators (HRSGs), EPRI, Palo Alto, Calif., 2006.
2. Buecker, B. Condenser Chemistry and Performance Monitoring: A Critical Necessity for Reliable Steam Plant Operation; from the Proceedings of the 60th Annual Meeting, International Water Conference, Pittsburgh, Pa., October 18–20, 1999.
Author

Brad Buecker is a process specialist with Kiewit Power Engineers (9401 Renner Boulevard, Lenexa, KS 66219; Phone: 913-928-7000; Fax: 913-689-4000; Email: [email protected]). He has 30 years of experience in, or affiliated with, the power industry, much of it in steam generation chemistry, water treatment, air-quality control and results engineering. He has held positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Co.’s La Cygne, Kansas station. He also spent two years at a CPI manufacturing plant. Buecker has an A.A. in pre-engineering from Springfield College in Illinois and a B.S. in chemistry from Iowa State University. He is a member of the ACS, AIChE, ASME, NACE and CTI (Cooling Technology Institute). He is also a member of the ASME Research Committee on Power Plant & Environmental Chemistry, the Electric Utility Workshop planning committee and the Coal-Gen planning committee.