This column is based on “Bio-Acrylic Acid Production from Glucose – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/acrylic-acid-production-cost.
Acrylic acid is a moderately strong carboxylic acid, primarily used in the production of emulsion and solution polymers. On a commercial scale, acrylic acid is mainly produced from the oxidation of propylene or propane derived from petroleum or natural gas — both nonrenewable fossil carbon-based starting materials. In recent years, significant research and development efforts have been made toward manufacturing acrylic acid from natural renewable starting materials, such as glucose or molasses originating from biomass, or from glycerol, a byproduct from biofuels production.
The process
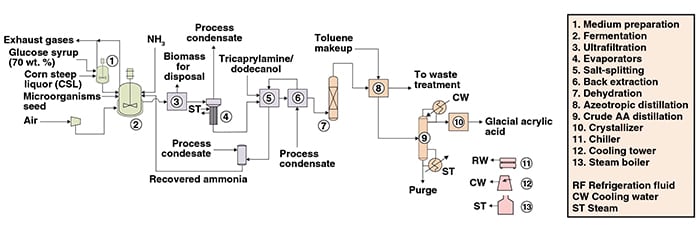
The following paragraphs describe bio-based acrylic acid production from glucose syrup, where the glucose is initially fermented to generate 3-hydroxypropionic acid (3-HPA), which is then dehydrated to produce acrylic acid. Figure 1 presents a simplified flow diagram of the process.
Fermentation. The culture media used in the pre-fermentation and in the fed-batch fermentation are prepared by diluting the glucose syrup (fermentable carbon source) and nutrients (source of nitrogen, vitamins and minerals) in water. The main fermentation is carried out in fed-batch mode and under aerobic process conditions. The pH of the fermentation broth is controlled by adding an ammonia (NH3) solution to keep the broth’s pH around 7. Due to the presence of the ammonium hydroxide, most of the 3-hydroxypropionic acid produced is neutralized to 3-hydroxypropionate, in such a way that the broth contains mainly the conjugate base of 3-hydroxypropionic acid.
Separation. After fermentation, cell debris and other suspended solids are removed from the broth by ultrafiltration. The permeate solution is pumped to evaporators, while the retentate — mostly biomass and water — is disposed.
After evaporation, the concentrated fermentation broth is fed to an agitated vessel operating under vacuum, along with tricaprylamine (organic solvent) and dodecanol (co-solvent). The mixture is heated so that the ammonium salt of the 3-hydroxypropionic acid splits, producing ammonia and 3-hydroxyproprionate (3-HP). The liquid stream from the salt-splitting is then back-extracted in countercurrent contact with hot water, yielding an aqueous phase rich in 3-HP and a 3-HP-lean organic phase.
The resultant aqueous solution of 3-HP is passed through a reactor column packed with γ-alumina catalyst, where the 3-HP is dehydrated to acrylic acid that is fed, in turn, to an azeotropic distillation system along with toluene — an azeotropic solvent for water removal.
Purification. The acrylic acid solution is fed to the crude acrylic acid distillation unit for removal of remaining impurities, and finally submitted to a melt crystallization carried out in multitube exchangers. After operating cycles (alternating the crystallizing and melting stages), glacial acrylic acid is obtained.
Economic performance
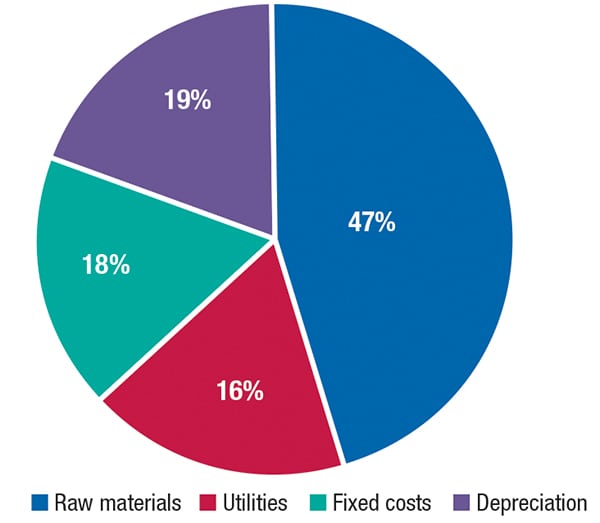
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated for making bio-acrylic acid was about $1,580 per ton of bio-acrylic acid in the fourth quarter of 2013. The analysis was based on a plant with capacity to produce 150,000 metric ton per year of bio-acrylic acid. A breakdown of the total operating cost of such a plant is presented in Figure 2.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.