Environmental regulations and high energy costs are driving technology advances that make plants cleaner and more efficient
For some, the production of chlorine and caustic has been considered a nasty business: not only are the products dangerous if not handled properly, but the traditional, century-old chlor-alkali process itself — the electrolysis of brine — has been carried out in hundreds of cells that either contain mercury (a toxic pollutant) or a diaphragm made of asbestos (a carcinogen). And continuously flowing through the banks of cells are thousands of Amperes of electrical current (current densities of 5–6 kA/m2), which makes the process one of the most energy-intensive in the chemical process industries (CPI).
Nevertheless, both Cl2 and NaOH, as well as the byproduct H2 are extremely important in just about all sectors of the CPI. In Europe, for example, over half of all chemicals produced rely on chlor-alkali products, with the vast majority of Cl2 used in modern plastics, such as polyvinylchloride (PVC), according to Euro Chlor (Brussels, Belgium; www.eurochlor.org). Also, 85% of all medicines made in Europe are based on chlorine, over 90% of European drinking water is made safe for consumption using chlorine and 25% of medical devices involve chlorine chemistry in their production, says Euro Chlor.
Because of the tremendous need for these products, and the fact that transporting huge volumes of Cl2 is impractical, hundreds of chlor-alkali plants are running throughout the world — close to where the Cl2 is needed — and will continue to be indispensible. What makes today’s chlor-alkali plants different from those of just a few decades ago, is that they are much more efficient than their predecessors — due to the introduction of bipolar membrane technology (Chem. Eng., February 2001, pp. 31–35). And more recently, the switch to so-called zero-gap technology, in which the gap between the cathode and the membrane is minimized, is pushing the efficiencies to nearly the thermodynamic optimum, says Jacqueline Oonincx, director Technology — Chlor-Alkali & Chloromethanes, Akzo Nobel N.V. (AkzoNobel; Amsterdam; the Netherlands; www.akzonobel.com). The company’s MEB plant in Rotterdam (Figure 1), for example, is currently being modernized to incorporate the latest zero-gap membrane technology — a process that takes several years to carry out.

Figure 1. AkzoNobel’s MEB (Membraan Electrolyse Bedrijf) plant in Rotterdam is one of the first and largest membrane-based chlor-alkali plants in Europe. The facility is now converting to the latest zero-gap membrane technology, which will significantly increase the energy efficiency
AkzoNobel
Closing the gap
The first “fully” zero-gap membrane cell was launched in 2012, when thyssenkrupp Uhde Chlorine Engineers (Dortmund, Germany; www.thyssenkrupp-uhde-chlorine-engineers.com) introduced its BM2.7v6 — the sixth generation of its single-element membrane cell. Like its predecessors, the cells have a membrane area of 2.72 m2, making it easy to upgrade existing units. The BM2.7v6 is said to have a zero gap over the entire active membrane. As a result, the BM2.7v6 reduces power consumption to 2,020–2,035 kWh per metric ton (m.t.) of NaOH at 6 kA/m2, says the company (see Table 1 for comparisons). The BM is being used in a number of commercial plants, including several recent conversions from mercury to membrane technology, such as AkzoNobel’s plant in Frankfurt am Main, Germany (250,000 m.t./yr of Cl2), which was completed in 2015. More recently (October 2016), CABB AG (Pratteln, Switzerland; www.cabb-chemicals.com) started up two electrolyzers, each with 131 sixth-generation, single-element cells from thyssenkrupp, thereby replacing the amalgam electrolysis plants. The new plant increases the Cl2 capacity from 27,000 m.t./yr to 47,000 m.t./yr, thereby meeting the site’s demands and essentially eliminating the need for Cl2 transports, says CABB.
“We are working continuously on improving our technologies; for example with new surface structures, membranes, materials or coatings,” says Denis Krude, CEO of thyssenkrupp Uhde Chlorine Engineers. “The main target is to lower the power consumption even further, under 2,000 kWh/m.t. NaOH,” he says.
Meanwhile, other membrane-electrolysis suppliers are also working to improve efficiencies of their own zero-gap technology. For example, in 2014, Bluestar (Beijing) Chemical Machinery Co. (China; www.bcmc.com) first commercialized its second-generation zero-gap electrolyzer technology, the Natural Circulation Bipolar Zero Gap Electrolyzer (NBZ-2.7II), which has a power consumption of 2,030 kWh/m.t. of NaOH. Since then, there are now 19 plants using this technology, says Jeff Pan, CCO and Overseas business director at Bluestar. The company is now working to further reduce the energy consumption to below 2,020 kWh/m.t. of NaOH with its NBZ-X electrolyzer, which is expected to be commercially available within the next two years, says Pan. It will be a skid-mounted plant that is easily installed, and the active electrode area will be smaller than the NBZ-2.7 II, he explains.
New membranes
In a related effort to reduce energy costs, new generations of ion-exchange membranes are also being developed. AGC Chemicals, a division of Asahi Glass Co. (Tokyo, Japan; www.agc-chemicals.com), has continued its efforts to reduce electric resistance of its ion-exchange membranes over the last 40 years. The company’s current “flagship” product, the Flemion F-8080, is a cation-exchange membrane (a fluorinated carboxylic polymer) that offers extremely low resistance and is said to be the preferred product by many users. The use of carboxylic polymer helps ion-exchange membranes in salt electrolysis achieve high electrical current efficiency.
At Euro Chlor’s 10th Technology Conference (May 16–18; Berlin, Germany), the company introduced the latest Flemion membranes and said it is working on a soon-to-be released version, the Fx-634, which is more robust than the F-8080 and F-8080A, and will enable a further reduction of the operating voltage by 50 mV, the company says.
Also at the the Berlin conference, Asahi Kasei Corp. (www.asahi-kasei.co.jp) gave an overview of its latest ion-exchange membrane technology, the Aciplex-F6800 series of perfluorosulfonic acid membranes, and previewed the next development, the F7001X, which will be commercialized in 2018. The F7001X features the company’s patented Ion Cluster Control technology, which is said to enhance the current efficiencies by 0.3–0.5% for the same cell voltage (CV). The F7001X has been shown to enable cell voltages to be reduced by 50 mV compared to the F6808.
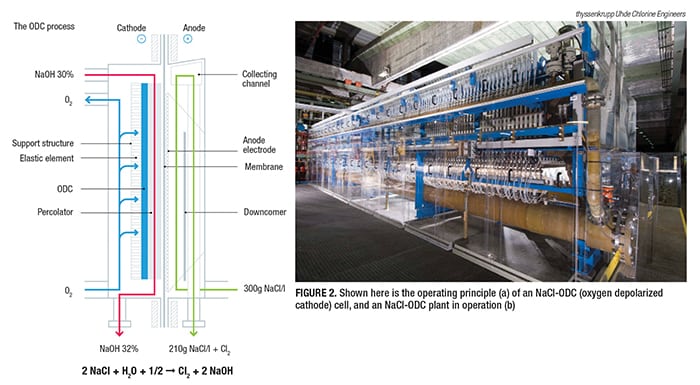
Oxygen depolarized cathodes
Possibly the biggest quantum jump in terms of energy reductions in the chlor-alkali production process has been the introduction of the oxygen depolarized cathode (ODC) technology (Figure 2). This magazine has been following this breakthrough since it was originally developed by Covestro AG (formerly Bayer MaterialScience; Leverkusen, Germany; www.covestro.com) and thyssenkrupp (for more details, see Chem. Eng., May 2007, pp. 50–55; www.chemengonline.com/articles.php?file=2007%2FCov%2FCov05012007_01.html, and Chem. Eng. December 2014, pp. 17–22; www.chemengonline.com/winning-with-electrochemistry). “This technology reduces power consumption by up to 25%, and it has already been implemented in several industrial plants worldwide since 2011,” says thyssenkrupp’s Krude.
The company also offers ODC technology for making Cl 2 from HCl, a process that promises to be an important way to recycle HCl generated in the production of methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI). “MDI and TDI production facilities are operating at extremely high rates due to the tight isocyanate markets, resulting in increased availability of HCl,” says Krude.
Mercury’s era comes to an end
Although the conversion of mercury cells to membrane cells can make good economic sense in terms of reducing operating costs (see Table 1), a number of mercury plants are still operating around the world, especially in Europe, where the mercury cells had traditionally been the technology of choice. Worldwide, there are still 34 mercury plants with a joint production capacity of 3 million m.t./yr of Cl2, according to Euro Chlor. At the end of 2016, membrane technology accounted for about 66% of European Cl2 production capacity, with mercury-cell’s share accounting for approximately 17% of the Cl2 production capacity, according to Euro Chlor’s “Industry Review 2016–2017,” published last month.
In 2017, conversion of mercury plants to membrane processes (or closures of the mercury plants) will further increase, says Euro Chlor. In 2013, best available technology (BAT) conclusions under the E.U.’s Industrial Emissions Directive implied that mercury cell technology can no longer be used in chlor-alkali units after 11 December 2017. On May 24 2017, the new E.U. regulation on mercury was published (EU 2017/852). The new regulation reinforces the phaseout deadline of the end of 2017, and Euro Chlor says it will continue to support the membership in identifying conversion and storage options. This regulation allows temporary storage of liquid Hg for a maximum of up to five years, with the possibility of extension of three additional years. Meanwhile, liquid mercury must be converted into mercury sulfide before being permanently disposed of, says Euro Chlor.
With the adoption of the EU Mercury Regulation in May 2017, the E.U. simultaneously ratified the United Nation’s Minamata Convention on Mercury (www.mercuryconvention.org). This ratification by the E.U. Commission prompted other Member States to act, bringing the number of ratifications up to the required 50 for the convention to enter into force on August 16, 2017. The Minamata Convention requires the phaseout of all mercury-cell chlor-alkali production by 2025.
The first Conference of the Parties (COP1) meeting was scheduled for September 24–27, 2017 in Geneva, Switzerland (after this article went to press), and the The World Chlorine Council (WCC; www.worldchlorine.org) said it would be attending. Established in 1993, WCC represents national and regional trade associations and their member companies, to promote best practices and the benefits of chlor-alkali chemistry.
In the U.S., the diaphragm cell is the dominant technology for its chlor-alkali sector. Of the two remaining mercury-cell plants still operating in the U.S., one will be converted to membrane technology by the end of this year, and the other is expected to close or convert by the 2025 deadline of the Minamata Convention.
In 2014, Ashta Chemicals Inc. (Ashtabula, Ohio; www.ashtachemicals.com) announced it would be investing $60 million to convert its chlor-alkali facility in Ashtabula, Ohio from mercury-cell to the BiChlor membrane electrolyzer of Ineos Technologies (London, U.K.; www.ineos.com). Ashta says the change will also result in a 20% reduction in energy consumption.
Staying competitive
Since 2007, there has been a gradual decline in European chlorine production; the chlorine industry has not followed the general growth of the chemical industry in Europe and the rest of the world, according to Euro Chlor. The regulatory and economic environment in Europe continues to challenge our global competitiveness, says executive director Dolf van Wijk. He points to statistics from Cefic (The European Chemical Industry Council; Brussels, Belgium; www.cefic.org) that show regulatory costs have doubled over the past decade, with energy policies continuing to increase power prices due to rising taxes and levies, reducing our global competitiveness,” he says. “Europe urgently needs to improve its policy support for the manufacturing sector to foster innovation, provision of new jobs, generate growth and supply the needs of future generations.”
Electricity accounts for at least 50% of the chlor-alkali production costs, says Ton Mandres, technical director at Euro Chlor. Whereas shale gas has essentially cut electricity costs in half in North America, energy is a bigger challenge for the E.U., he says.