Biodiesel, the methyl ester of fatty acids, has been accepted as a renewable-sourced alternative to fossil-derived diesel fuel. Today in many parts of the world, biodiesel is already blended with diesel fuel up to about 5%. Presently the demand for biodiesel is only approximately 40–50% of the production capacity, but the global biodiesel market is expected to experience strong growth in the next few years as the economy rebounds and the demand for renewable resources continues to increase.
To further improve the quality of the biodiesel and enhance the position of biodiesel as a viable, cost-effective renewable-energy source, there has been a continued effort to make further technology improvements. One area of focus has been to reduce feedstock costs. This is because typically raw material prices account for 90% of the biodiesel cost. It is also anticipated that raw material prices will further increase as the demand for biodiesel fuel grows. In this current economic climate, producers of biodiesel have turned to less expensive crude feedstocks, recycled feedstocks, and animal fats, which have recently gained popularity, for use in producing biodiesel. These raw materials contain varying levels of fatty acids. These fatty acids cause issues during the transesterification reaction necessary to produce biodiesel from triglycerides. Pretreatment of these feedstocks is frequently necessary to remove or react the fatty acids. Esterification has proven to be an effective technology to handle the fatty acids and increase the overall product yield.
Another area where technology improvements have been made entails improving the quality of the biodiesel, since there are very tight ASTM/EN specifications on impurities in the final biodiesel (such as phosphorous).
Two approaches have been developed and have proven to be effective; namely, purification of either (a) the feedstock or (b) the final product. The first approach has been developed within the past year as manufacturers have been increasingly interested in removing the impurities upfront in the manufacturing process, thereby limiting the negative impact these impurities may have against their equipment, process control, and catalytic processes. The second approach, purification of the final product, was established more than five years ago and has proven to be very effective at removing impurities from biodiesel generated from a variety of feedstocks.
The aforementioned technology improvements enable the use of lower cost triglyceride oils that are high in fatty acids and potentially contain other impurities. These improvements also result in a cost-effective route to produce within-specification biodiesel through the addition of relatively simple steps for the purification of the triglyceride oil and esterification of the free fatty acids prior to transesterification.
Physical refining
• Extracting. Removal of the oil from the beans, either by hexane extraction or physical squeezing of the bean to release the oil
• Degumming. Treating the extracted oil with phosphoric acid (or other weak acid), thereby converting the non-hydratable phosphatides into hydratable phospholipids. After phosphoric acid treatment, the mixture is subjected to base and water to remove the phospholipids and soaps via liquid/liquid extraction
• Bleaching. Removal of potential peroxides or peroxide formers, or both, and residual impurity removal by processing oil through diatomaceous earth filtration
• Deodorizing. Typically done as a final polishing step using steam distillation of low-boiling odor components. This step is often reserved for food grade applications
| Table 1. Materials Removed at each Step of the Pretreatment Process | |
| Step | Components Removed |
| Degumming | Gums, phosphatides, glycolipids, phospholipids and proteins. |
| Bleaching | Pigments (caratenoids and chlorophylls), soaps/FFA and polycyclic hydrocarbons |
| Deodorization | FFA, flavors, peroxides and degradation by-products, organic chloride containing pesticides, sterols and tocopherols |
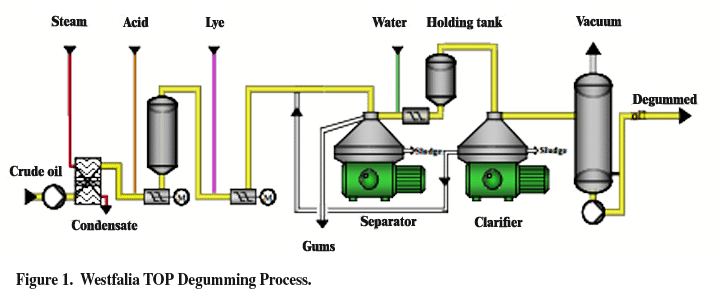
Not every step is necessary to produce a feedstock for the biodiesel industry. Simple physical refining by degumming the oil, followed by a method for polishing may be adequate for the biodiesel industry. Westfalia, for example, has a process known as TOP-degumming. The process involves partial degumming, namely, treating the feedstock with phosphoric acid, and only enough base for neutralization of phosphoric acid, but not carboxylic acid, to allow for retention of the FFAs. Figure 1 below shows a schematic of the proposed TOP degumming.
Chemical nature of impurities found in oils
There are a variety of impurities present in crude oil materials, dependent on their source. These materials may cause issues in the biodiesel process, including reactor fouling, catalyst poisoning, separation issues post-reaction, and failure to pass ASTM specifications. We classify these materials as either chemical fouling agents, or physical fouling agents.
Impurities removal from feedstocks
As discussed above, physical refining of oils will reduce the level of impurities to parts per million (ppm) levels. Unfortunately, this is not adequate for long term biodiesel manufacture, and biodiesel suppliers are constantly looking for ways to reduce impurities, with a focus on phosphorous removal. Phosphorous contamination in oils can come from two separate sources, the phospholipid impurities present in the oil after processing, and phosphoric acid and salts that remain after incomplete separation after degumming or from bone meal byproducts found in animal fats. Depending on the nature of the phosphorous impurity, it can be removed via an ion-exchange or an adsorption mechanism. Scientists have discovered Ambersep BD19WET as the preferred media for phosphorous removal. Table 2 below shows impurity removal effectiveness from three different commercially available oils; in each case, the samples of oil were passed through a column containing the methanol-dewatered resin. Unfortunately, the identity of the impurities in each feedstock is difficult to obtain.
| Table 2. Summary of Percentage analyte Removal from Various Oils | |||
| Analyte | Low level of impurity (animal fat) | Medium level of impurity (Jatropha oil) | High level of impurity (crude vegetable oil) |
| P | 80% | 77% | 30% |
| K | 80% | 95% | 65% |
| Na | 66% | 80% | 50% |
| Ca | 67% | 35% | 45% |
| Mg | 80% | 30% | 50% |
| Fe | 90% | 45% | 45% |
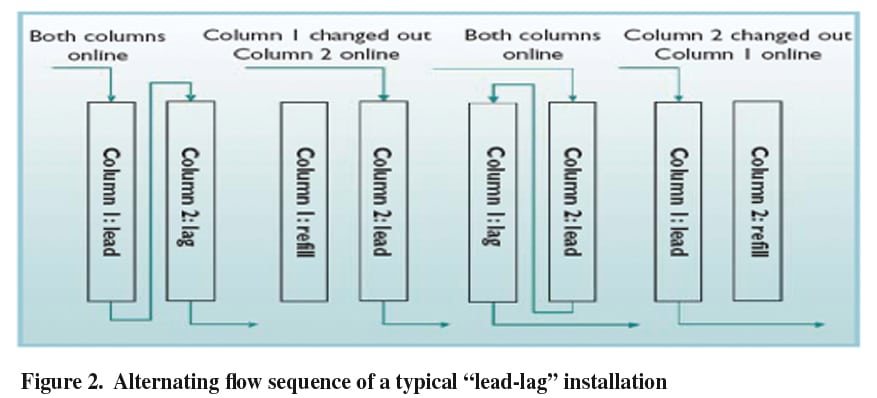
In a typical reactor system, a fixed bed column is used to house the resin. Temperatures necessary for this process are mild, and only need to be high enough to ensure proper flow of oil; which in some cases can be solid at ambient conditions. The column is designed to contain the appropriate volume of resin necessary to remove the impurity from the oil for the requisite amount of time. We suggest a length-to-diameter ratio of approximately two with a 10% freeboard. To optimize production, we recommend a lead-lag configuration (Figure 2). An example of a commercial unit is seen in Figure 3 below.

Impurities removal from biodiesel
There are many commercial biodiesel production processes and the design and construction of biodiesel facilities vary widely, but the base-catalyzed production of biodiesel generally occurs by mixing the alcohol and catalyst with the oil. The catalyst is typically sodium methoxide (methylate), hydroxide (caustic soda), or potassium hydroxide (potash). The reaction mixture is heated just above the boiling point of the alcohol (around 70°C) to speed up the reaction and the reaction time varies from 1 to 3 h. Excess alcohol is normally used to ensure total conversion of the fat or oil to the esters. Care must be taken to monitor the amount of water and free fatty acids in the incoming oil. If the free fatty acid level or water level is too high, it may cause problems with soap formation and the separation of the glycerol byproduct downstream. Once the reaction is complete, two major products exist: crude glycerol and crude biodiesel. Each has a substantial amount of the excess methanol that was used in the reaction. This excess methanol is removed by flash distillation. After methanol removal, two distinct layers result. The glycerol phase is much denser than the biodiesel phase and the two can be gravity separated with glycerol simply drawn off the bottom of the settling vessel. In some cases, a centrifuge is used to separate the two materials more quickly. Once the glycerol and biodiesel phases have been separated, the residual alcohol in each phase is removed by a flash evaporation process or by distillation; the alcohol is recovered using distillation equipment and is reused. Once separated from the glycerol, the biodiesel often contains residual alkali metals from the catalyst, alkaline earth metals from either the oil source, water (hardness) used in processing, residual soap and FFA material, and residual glycerol. To meet ASTM/EN specifications, this crude biodiesel must be further processed to remove these impurities. Ion-exchange resin technology is well suited for this purpose. The materials are designed to remove unwanted ions from a variety of materials, including water and a wide range of organic streams. The glycerol produced as a byproduct contains unused catalyst and soaps that are typically neutralized with an acid and sent to storage as crude glycerol. This crude glycerol can be upgraded by either distillation or chromatography.
Recently, the ASTM test method for biodiesel has been upgraded to include cold soak filtration. This test was developed as a surrogate test to help predict the cold temperature performance of biodiesel fuel. The test involves cooling the biodiesel overnight, and then allowing the material to return to room temperature. The material is then filtered by ASTM specified techniques to an either pass or fails endpoint. Researchers at Archer Daniels Midland have found that monoglycerides, sterolglucosides, soap (fatty acid), and water are culprits in a failed cold soak test. Figure 4 below shows the structural similarity of these impurities.
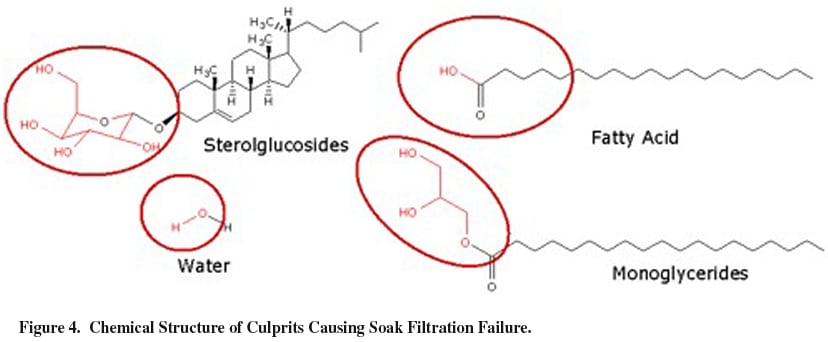
As can be seen above, a common structural characteristic of these impurities is the hydroxyl, or polyhydroxyl moiety. We have developed a resin to remove these polyhydric materials. The figure below shows the results of specific targeted research surrounding resin optimization for monoglyceride removal. For these experiments, monoglyceride was added to a stock solution of biodiesel, to a level of 1% monoglyceride. The resultant fuel was subjected to ion-exchange resin, and monoglyceride removal efficiency was measured by gas chromatography (GC) analysis (ASTM D-6584). Of the 30 resins tested, approximately ten resins showed promise for this application. Clearly, resin microstructures are responsible for performance, be it from extended gel phase of the polymer, hydrophile/lipophile balance of adsorption pockets, or pore size. These effects are currently under study.
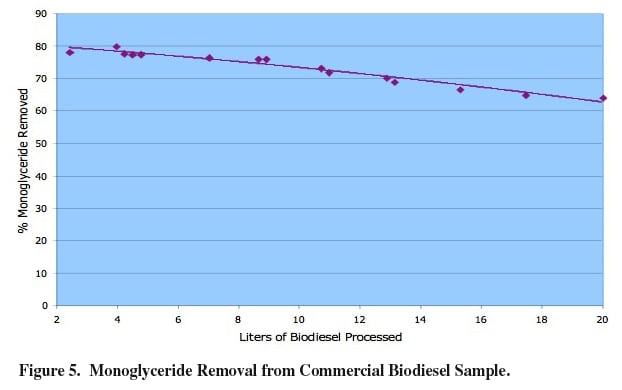
Extending the testing to commercial examples of biodiesel showed similar performance, with ~90% of the monoglyceride being removed by simply passing the fuel through a column containing Ambersep BD19DRY resin. In the example below (Figure 5), commercial oil containing approximately 0.30% monoglyceride was passed through a column containing the optimal resin necessary to remove monoglyceride. Sample aliquots were collected, and percentage of monoglyceride removal was calculated. After the resin is saturated with monoglyceride, a simple methanol wash to remove the monoglyceride is recommended, after which time, the resin is placed back in service to once again remove monoglyceride for the biodiesel fuel. This regeneration scenario can be carried out numerous times, and the lifetime of the resin will be solely dependent on the amount, and nature of impurities found in the specific biodiesel sample.
Summary and future direction
Presently the market for biodiesel is relatively weak, as the demand is 40–50% lower than the production capacity. However, as the interest in renewable resources continues to increase and the economy rebounds, it is expected that biodiesel will continue to grow. Furthermore, advancements in the technology to produce biodiesel have been made, which will enable the use of less costly triglyceride oils. This will greatly improve the cost position for biodiesel, as well as the quality of the final methyl ester product. The purification technologies described above will likely play a significant role in the growth of the biodiesel market.
Robert J. Olsen is an applications specialist, industrial process — catalysis at Dow Water & Process Solutions (Phone: 215-619-5422).