This column is based on “Cyclohexane from Benzene and Hydrogen – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/cyclohexane-production-cost.
Cyclohexane is a relatively stable cycloalkane, present in crude oils in concentrations of 0.1–1.0%. This cycloparaffin is a colorless, flammable liquid, widely used as an intermediate in nylon manufacturing. On a commercial scale, cyclohexane production is almost entirely based on the catalytic hydrogenation of benzene, which can be conducted in the liquid or vapor phase in the presence of hydrogen.
The process
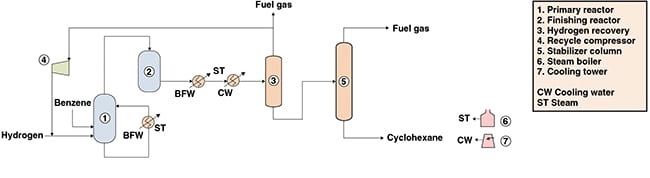
The following paragraphs describe a process for the production of cyclohexane from benzene and hydrogen that involves liquid-phase hydrogenation of benzene in the presence of a nickel-based catalyst. Figure 1 presents a simplified flow diagram of the process.
Reaction. Initially, benzene is fed to the primary reactor along with fresh and recycled hydrogen. The hydrogenation reaction is carried out in a bubble column reactor, in the presence of a nickel catalyst. The catalyst is maintained in a suspension with the aid of an external circulation loop. Most of the heat of reaction is removed by the vaporization of the product stream, which is further recovered via a top stream within the reactor. The remaining reaction heat is removed in the external loop, by passing the reactor reflux stream through a heat exchanger against boiler feedwater, producing low-pressure steam.
Most of the benzene feed is converted in this step. The top gaseous product stream is directed to a fixed-bed reactor, where the remaining benzene content is converted to cyclohexane. The finishing hydrogenation reaction is conducted in the presence of a solid nickel-based catalyst supported on alumina.
Hydrogen recovery. The product stream is fed to a knock-out drum operated at high pressure. Most of the cyclohexane in the feed condenses, producing two streams: one gaseous hydrogen-rich stream and a liquid cyclohexane-rich stream. The hydrogen stream is routed to the recycle compressor, where it is compressed to the pressure of the primary reactor and recycled.
Purification. In a distillation column, the liquid cyclohexane-rich stream that is recovered is stripped of lighter contaminants, such as methane, ethane and soluble hydrogen. Light-ends recovered from column’s top are used for fuel, while a cyclohexane stream with a residual benzene content lower than 100 ppm is obtained from column’s bottom.
Production pathways
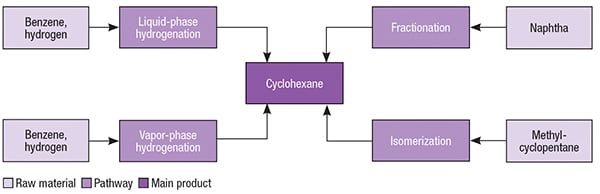
Cyclohexane has been primarily manufactured by the hydrogenation of benzene, which can be carried out in both liquid and gaseous phases, in the presence of different metal-based catalysts. This chemical may also be obtained from naphtha fractionation, or by isomerization of methylcyclopentane. Different pathways for cyclohexane are presented in Figure 2.
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce cyclohexane was about $1,400 per ton of cyclohexane in the first quarter of 2014. The analysis was based on a plant constructed in the U.S. with capacity to produce 200,000 metric ton per year of cyclohexane.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.