Uncontrolled exothermic reactions present significant safety risks, including over-pressurization of a reactor. Presented here is information about how runaway reactions occur, and what can be done to avoid them
Synthesis reactions are a cornerstone of chemical manufacturing and engineering. Due to the exothermic nature of many such reactions, a common concern exists: a runaway reaction scenario. Through a variety of potential causes or mechanisms, uncontrolled exothermic reactions can lead to an uncontrolled energy release. During a runaway, a tremendous amount of heat can be generated. However, temperature alone rarely leads to a risk to people, equipment, or the environment. The pressure effects of the runaway reaction are the primary safety risk. Over-pressurization of a reactor or other vessel can occur quickly when the temperature rises uncontrollably.
While pressure and temperature are inexorably linked, the pressure effects observed during seemingly similar runaways can result in significantly different safety outcomes. By understanding the driving forces behind these pressure effects, the safety risks can be effectively managed.
Pressure-rise causes
During a runaway reaction, pressure rises rapidly due to one or more of the following causes:
- Reaching the boiling point of the solvent used, leading to a rapid increase in vapor pressure
- Reactants, products and intermediates begin to breakdown, releasing significant energy, permanent gas or both
- Permanent gas normally generated by the reaction reaches a generation rate beyond what the vessel can withstand
Vapor pressure. Most synthesis reactions use a solvent. The solvent used has a variety of impacts on a reaction, but during a runaway, the boiling point (BP) of the solvent is critical. As the reaction mass nears the boiling point of its solvent (or low-BP reactants), the pressure can rise dramatically. For reactions occurring in an enclosed vessel, once the solvent begins to boil, the pressure can easily reach the maximum allowable working pressure (MAWP) of the vessel (Figure 1). In this case, controlling temperature is the means to controlling over-pressure.

FIGURE 1. If reaction temperatures exceed solvent boiling temperatures, pressures in enclosed vessels can reach maximum allowable working pressure
Temperatures rising above the solvent boiling point can pose an additional challenge for relieving the pressure during an over-pressurization event. Once the relief vent opens, the formation of vapor bubbles due to boiling in the liquid can cause rapid expansion of the reaction mass, leading to two-phase flow. Two-phase flow is when the relief system has both liquid and gas discharging. This can significantly reduce flow capacity of the relief device or piping, allowing the pressure in the vessel to rise despite the relief device opening. Two-phase flow requires larger-diameter relief systems than either pure liquid or vapor flow. A small-scale example of two-phase flow is opening a shaken soda can or bottle: the carbon dioxide rushes out, taking the soda with it and spraying the contents out of the bottle.
Secondary or decomposition reactions. Often, the pressure effects of the runaway of an intended reaction can be contained. However, as the temperature rises, unintended secondary reactions may be initiated. These secondary reactions are often more energetic than the original intended reaction. This poses two main risks. First, the temperature can climb dramatically, allowing the reaction to reach the solvent boiling point and beyond. Second, the materials in the vessel can decompose at these elevated temperatures.
Decomposition reactions release significant energy and can release permanent or non-condensable gas. Permanent gas generation can add to the vapor pressure generated during the reaction.
Permanent gas generation. Certain reactions generate non-condensable or permanent gases as a part of the intended reaction. As a rule of thumb, the reaction rate doubles for each 10°C increase in temperature (based on the Arrhenius equation). Uncontrolled heating will drive exponential increase in the rate of gas generation. Gas generation can build on the impact of vapor pressure and secondary decomposition reactions. All three pressure mechanisms must be considered for this kind of reaction, and how they influence each other.
While not covered in this article, gases generated by decomposition are often flammable or oxidizing. This can increase the risk of an ignition in the headspace of the pressure vessel, leading to rapid and dramatic over-pressurization. API 521 [1] has information on handling these hazards.
Hazard assessment
Systematic assessment of reaction hazards is key to scaling reactions up or introducing new reactions into existing processing equipment (Figure 2). Ultimately, it comes down to asking two key questions:
- Is this runaway reaction likely to lead to over-pressurization?
- Does the process have a robust basis of safety for over-pressurization scenarios?

FIGURE 2. Systematic assessments of chemical reaction hazards help determine whether a reaction is likely to lead to over-pressurization, and whether the process has a robust basis of safety for overpressure situations
There are systematic ways to evaluate a reaction for the over-pressurization potential it may pose. One system uses criticality classes for classifying reactions. Sometimes known as Stoessel classes, this system is fully summarized in “Thermal Safety of Chemical Processes” [2]. The system uses four temperatures to categorize the risk posed by a potential reaction runaway. First is the process temperature, or the temperature at which the runaway would begin. Second is the maximum temperature of synthesis reaction (MTSR). A runaway reaction depends on the chemical fuel available to it. The MTSR is the temperature the reaction mass would reach once the runaway had exhausted itself. The MTSR is only for the intended reaction and does not consider the temperature that could be reached if a secondary reaction were initiated.
For these secondary reactions, there is a temperature that, once reached, the secondary reactions will fully runaway within 24 hours. This is known as the temperature of time to maximum rate – 24 hours (Td24).
Last is the maximum temperature for technical reasons (MTT). This temperature depends on the system and can vary for the same reaction run in different equipment. MTT should be picked based on the technical limit for the specific equipment to be used. This is often the temperature at which the vapor pressure of the system exceeds the MAWP of the vessel, or the set pressure of a relief device.
By using these four temperatures, the criticality class of the reaction can be assigned. A rating of 1 poses the lowest risk, while a rating of 5 poses the highest risk. In its essence, if the MTSR is higher than the MTT or Td24, the criticality goes up.
Take for example, a reaction with a MTSR of 100°C, a Td24 of 150°C, and temperature at the rupture disk setting (MTT) of 120°C. This would be a critically class of 1. If the intended reaction ran away, it would only reach 100°C. This is below the technical limitations of the vessel at 120°C and below the temperature that would likely trigger a secondary reaction.
Alternatively, consider a reaction with MTSR of 150°C, a Td24 of 100°C, and rupture disk setting reached at 120°C (MTT). The MTSR would be sufficient to initiate secondary reactions, further driving temperature and pressure effects. This occurs prior to the venting of the vessel. The rupture disk would need to be sized to accommodate the pressure effects of the main and secondary reactions. This is a criticality class of 5.
To properly assess a reaction for criticality, the four key temperatures must be known. The initial runaway temperature should be identified by the process hazard analysis (PHA) for the reaction (more information on PHAs is provided later). The MTSR is a function of several parameters, such as initial temperature, failure mode and the reaction itself. The MTSR is often identified by reaction calorimetry. The Td24 depends on the thermal stability of reactants, products and intermediates used in the reaction. This is commonly identified using calorimetry testing. The Td24 and MTT are influenced by a number of factors, so care should be taken to ensure that the values are conservative.
By knowing the criticality class, decisions can be made on the process design and basis of safety, accordingly. Given the number of inputs that impact MTSR, Td24 or MTT, the criticality class of a reaction is not fixed, and adjustments can be made to lower the criticality class. For example, because the MTSR is a function of the temperature at which the runaway begins, running the reaction at a lower temperature would reduce the MTSR.
Tools for novel reactions
While criticality classes allow for the systemic assessment of runaway reactions, they require information and data that may not be available for novel reactions. There are other tools and methods available to help identify reactions that may fall into higher criticality, and thereby pose over-pressurization risk.
A review of the chemistry can be insightful when it comes to assessing materials. Certain functional groups have higher decomposition energies. A summary of the decomposition energy for various functional groups and chemical structures can be found in Table 1 [3]. The presence of high-energy functional groups, or multiple structures containing stored chemical energy, indicate that if the Td24 is reached, the material could release significant energy, driving pressure increase. Additionally, molecules with complex structures pose a higher risk of permanent gas generation once Td24 has been reached and a decomposition begins. Certain chemical families, such as peroxides, are known to generate permanent gas during a decomposition. For critical safety assessments, such as identification of decomposition products, the safety data sheet (SDS) should be read with care as not all SDSs are created equal [4]. Information on chemical reaction hazards found on the SDS should be validated using other means where possible. Another useful resource for identifying unstable materials with low Td24s is NFPA 400 Annex B [5].
Software can be leveraged to lighten the load. One commonly used software is the chemical thermodynamic and energy release program (CHETAH) [6]. This software is produced by ASTM International and can model chemical reactions as well as the decomposition of materials. Modeling the heat of reaction can help with determining the likely MTSR for a reaction if the heat of reaction and the specific heat capacity of the reaction mass are known.
CHETAH also can model how energetic a material’s decomposition would be. Multiple properties are calculated including maximum heat of decomposition. For complex reactions and molecules though, CHETAH analysis can have a significant margin of error [7]. As such, CHETAH analysis alone should not be used to assess the criticality of a reaction. Instead, software tools such as CHETAH are often used to guide reaction calorimetry to ensure a targeted yet comprehensive test plan.
Calorimetry
The final critical tool for risk assessment is reaction calorimetry. Three common methods are discussed here: differential scanning calorimetry (DSC), adiabatic calorimetry and isothermal reaction calorimetry.
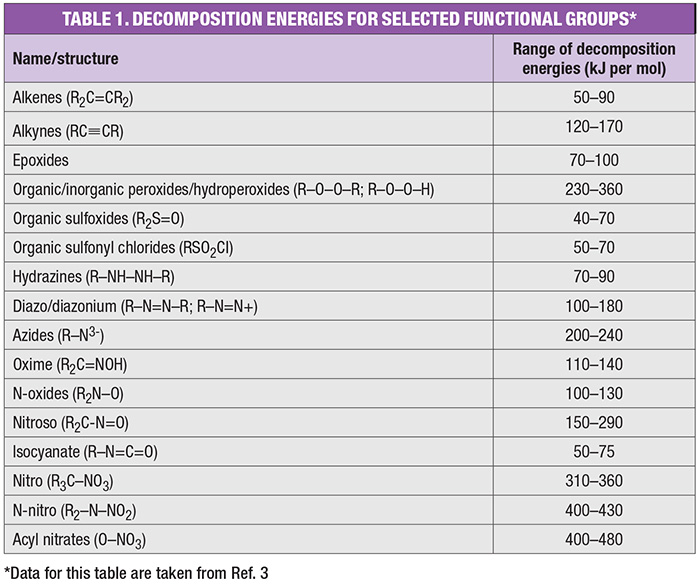
Differential scanning calorimetry. DSC testing is extensively used for a variety of applications (Figure 3). The testing identifies exothermic and endothermic reactions using minimal sample size [8]. As such, DSC testing can provide valuable data without requiring extensive sample size, time or resources. For exothermic reactions, DSC can provide data on heat of decomposition and onset temperature. One of the key drawbacks is that a larger safety margin is needed when using the onset temperature. Pressure generation data are also not recorded by DSC testing.
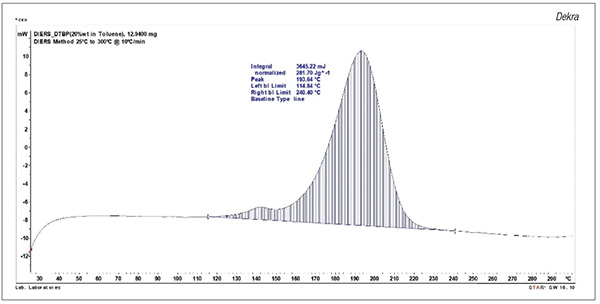
FIGURE 3. Differential scanning calorimetry (DSC) testing, such as the plot shown here for di-tert-butyl peroxide, provides valuable data with small sample sizes (graph developed by Dekra)
Adiabatic calorimetry. If a more accurate onset temperature or pressure generation data are needed, adiabatic calorimetry is the next step. Adiabatic reaction calorimetry, such as accelerating rate calorimetry (ARC) [9] is any heat generated by the sample is not lost to the testing environment (Figure 4). This more closely simulates large chemical processes in plants and enables more accurate onset temperatures for secondary reactions. ARC testing also captures pressure generation data, including maximum rate of pressure increase and permanent gas generation. These data are critical in determining an accurate Td24 for a reaction. ARC testing is a quantitative way to assess the pressure effects of a secondary reaction.
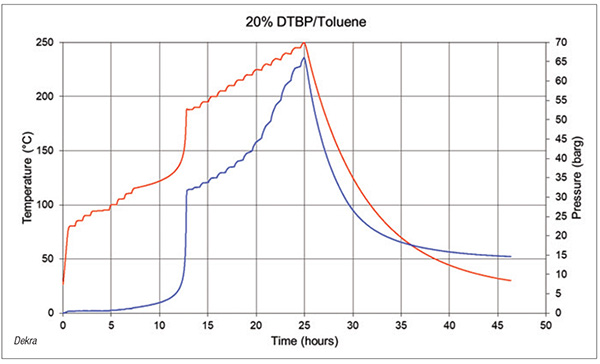
FIGURE 4. Accelerating rate calorimetry (ARC) is a type of adiabatic calorimetry that produces graphs such as the one shown here for di-tert butyl peroxide (DTBP) in toluene (graph developed by Dekra)
Isothermal reaction calorimetry. While DSC and calorimetry help refine the Td24 for a process, knowing the heat of reaction and associated MTSR is vital for determining the criticality. There are specialized reaction calorimeters that can precisely measure the heat of reaction of the intended reaction. This combined with the specific heat capacity provides the adiabatic temperature rise or the maximum temperature the reaction mass could reach assuming no heat loss to the environment. MTSR can be calculated using this value, along with the onset temperature of the runaway.
Basis of safety
Using all these tools and experiments provides a holistic and systematic assessment of the risk posed by a runaway reaction. With this information, a robust basis of safety for protecting against the pressure effects can be developed.
With a systematic approach in place to guide hazard identification and assessment, the next step is to ensure appropriate mitigation and control of pressure buildups. This is where the basis of safety comes in. A basis of safety is a summary of the key hazards of a process, along with the controls and processes in place to manage and mitigate these hazards [10]. For over-pressurization of vessels, it requires the knowledge of the mechanisms of pressure generation. Process controls and devices can then be identified and designed specific to the relevant process. These should be balanced between those that prevent a runaway from occurring (controls, interlocks and so on), and those that mitigate the effects if a runaway reaction initiates (quenching, emergency relief and so on). The first element, knowledge of the hazards, is essential. There have been accidents where a relief device for a pressure vessel existed, but it was not adequately sized for the event that occurred [11].
Much like criticality classes, there are systematic ways to approach hazard identification for chemical processes. The process hazard analysis (PHA) is the typical approach. A PHA uses various methodologies to assess a process for what could go wrong, and what the outcome of that scenario could be. Pertinent to this discussion, a PHA will help identify what events could result in a runaway reaction or rapid pressure generation. Some common scenarios include the following:
- Failed cooling
- Loss of agitation
- Incorrect addition or incorrect order of additions
- Temperature sensor fouling/failure
- Pool fire or external heating
A PHA is typically conducted using a cross-functional team to ensure the scenarios are thoroughly assessed. For scenarios with potentially catastrophic outcomes, inherently safer design principles are used when possible. An example of this would be to specify a higher-MAWP vessel, thereby allowing a higher MTT, and reducing the criticality class for the reaction. Sometimes such changes are not viable, and layers of protection can be used to ensure redundancy in key safety systems.
While all runaway reaction scenarios pose risk, pool fires or external heating are different from the rest. Pool fires can occur when flammable liquids collect under a reactor and catch fire. This provides an external heat source to the vessel contents. The MTSR calculations assume heat is not lost or gained to the environment. A pool fire adds heat to the system. External heating means the MTSR is no longer a reasonable worst-case temperature the reaction mass could reach. If a pool fire is a credible scenario, adiabatic calorimetry can provide a quantitative way to assess the pressure generation that would occur.
Emergency relief systems
Total elimination of over-pressurization risk is often not possible. This does not preclude reliable operation of the process, as a strong basis of safety includes multiple controls and methods. Controlling and reducing the likelihood of a runaway reaction requires its own set of controls [2, 3]. This article focuses on how to manage and mitigate the pressure effects of the runaway. For reactions with lower MTSRs and high Td24, it may be possible to ensure the vessel’s MAWP is high enough to contain the vapor pressure generated during a reaction. If preventing the temperature from reaching the Td24 is not reliable, then two main options exist.
The first option is pressure relief via an emergency relief system (ERS) [12]. For systems that do not use reactive chemistry, there are a variety of resources to help guide relief sizing [13]. The risk with these, known as non-reactive systems, is mainly centered around vapor pressure generated during an external fire. For reactive systems, the sizing of an ERS is more complex [12]. These relief devices may be required to handle vapor pressure, non-condensable gases, two-phase flow and hazardous by-products. Adiabatic calorimetry, such as the Vent Sizing Package II™, is used to guide design of these devices. Equipment downstream of the relief device must be considered as well. If the ERS is connected to a long run of piping, this can reduce the effectiveness of the ERS. Catch tanks, scrubbers and flares can also reduce the flow capacity of an ERS. Additional resources for ERS design are available through the Center for Chemical Process Safety [14]. The Design Institute for Emergency Relief Systems (DIERS) is a user group organized by the American Institute of Chemical Engineers (AIChE; New York, N.Y.; www.aiche.org) that guides the latest in ERS design. DIERS can be a valuable resource for those dealing with reactive system ERS [15].
Even with the most advanced ERS, some rates of pressure increase can be more than a reasonably sized ERS can handle. This can mean vessel deformation is possible during a relief scenario. For other cases, the maximum rate of pressure increase is too great and even with venting, vessel failure will occur. While this is a rarer scenario, a basis of safety can be built with this in mind. Options such as quenching, dumping and injection of a reaction poison should be considered. The area surrounding the vessel should be assessed for the consequence of a vessel failure. Known as a facility siting study, the outcome of the study can guide actions to protect operators and the surround facility. PHAs include a facility siting element, commonly using a checklist approach. For vessel failure scenarios, more comprehensive studies are done to better quantify the risk and actions to be taken.
The risk of over-pressurization in synthesis reactions can rarely be eliminated. A wide variety of tools and resources are available for use, and for a reason. However, they all have limitations and draw backs. Over-reliance on a singular tool or method will miss novel hazards. The best reviews build a “clinical picture” of the hazards. They pull together information from a variety of sources to ensure the risk is well defined, then the diagnosis or appropriate actions can be taken. By building the complete picture, over-pressure risk for synthesis reactions can be well managed.
Edited by Scott Jenkins
Acknowledgement
The author would like to recognize the review and valuable input to the article of John Wincek.
References
1. American Petroleum Institute, API standard 521, Pressure-Relieving and De-pressuring systems, American Petroleum Institute, www.api.org/~/media/files/publications/whats%20new/521%20e6%20pa.pdf.
2. Stoessel, F., “Thermal safety of Chemical Processes: Risk Assessment and Process Design,” Wiley-VCH, 2020.
3. Rowe, S., Chemical Reaction Hazards and Thermally Unstable Substances: A Strategic Guide to Reaction Hazard Assessment, Dekra, 2018, www.dekra.us/en/chemical-reaction-hazards-and-thermally-unstable-substances/.
4. Kolchinski, Alexander G., When Safety Data Sheets are a Safety Hazard, Organic Process Research & Development, 26 (2), 263-266, 2022, DOI: 10.1021/acs.oprd.1c00427.
5. National Fire Protection Association, standard NFPA 400, Hazardous Material Code, NFPA, www.nfpa.org.
6. ASTM International, Chemical Thermodynamic and Energy Release Program. The ASTM Computer Program for Chemical Thermodynamic and Energy Release Evaluation (CHETAH®). https://www.chetah.org/.
7. Young, K. L., Humes, J., Justice, C. and Mentzer, R., Experimental versus predicted heats of reaction for some common reaction types in the pharmaceutical industry. Organic Process Research & Development, 26(11), 3,130–3,140, 2022.
8. ASTM International, Test Method for the Thermal Stability of Chemicals by Differential Scanning Calorimetry. ASTM E537-20 https://doi.org/10.1520/e0537-20.
9. ASTM International, ASTM E1981, Standard Guide for Assessing the Thermal Stability of Materials by Methods of Accelerating Rate Calorimetry, ASTM International, www.astm.org.
10. Wincek, J.C., Basis of safety: A concise communication method for critical process safety information. Process Safety Progress, 30(4), 315–318, 2011. doi.org/10.1002/prs.10471.
11. U.S. Chemical Safety and Hazard Investigation Board (CSB), T2 Laboratories Inc. Reactive Chemical Explosion, CSB, 2009. www.csb.gov/t2-laboratories-inc-reactive-chemical-explosion/.
12. Dekra SE, Emergency Pressure Relief Systems, self-published whitepaper, www.dekra.us/en/design-of-emergency-pressure-relief-systems/.
13. Center for Chemical Process Safety (CCPS), “Guidelines for Pressure Relief and Effluent Handling Systems,” Wiley & Sons, American Institute of Chemical Engineers (AIChE), www.aiche.org. 2017.
14. Dekra SE, Some Common Pitfalls in Design Emergency Vent Systems, self-published whitepaper, www.dekra.us/en/some-common-pitfalls-in-designing-emergency-pressure-relief-systems/.
15. Design Institute of Emergency Relief Systems (DIERS), AIChE, www.aiche.org, 2025.
Author
 Bradley Silva is a chemical engineer supporting DEKRA’s (1000 Town Center Drive, Suite 600, Oxnard, CA 93036; Email: bradley.silva@dekra.com; Website: dekra.us/process-safety) Process Safety Services. With a background in chemical engineering and process safety management, Silva brings specialized experience from the aviation and specialty chemical industries. At DEKRA, Silva focuses on reactive, energetic and flammable processes and materials, supporting reaction-calorimetry testing projects for U.S. and global partners. He also assists international clients with risk-based hazard management tools. Silva holds a B.S.Ch.E. degree from the University of Alabama.
Bradley Silva is a chemical engineer supporting DEKRA’s (1000 Town Center Drive, Suite 600, Oxnard, CA 93036; Email: bradley.silva@dekra.com; Website: dekra.us/process-safety) Process Safety Services. With a background in chemical engineering and process safety management, Silva brings specialized experience from the aviation and specialty chemical industries. At DEKRA, Silva focuses on reactive, energetic and flammable processes and materials, supporting reaction-calorimetry testing projects for U.S. and global partners. He also assists international clients with risk-based hazard management tools. Silva holds a B.S.Ch.E. degree from the University of Alabama.