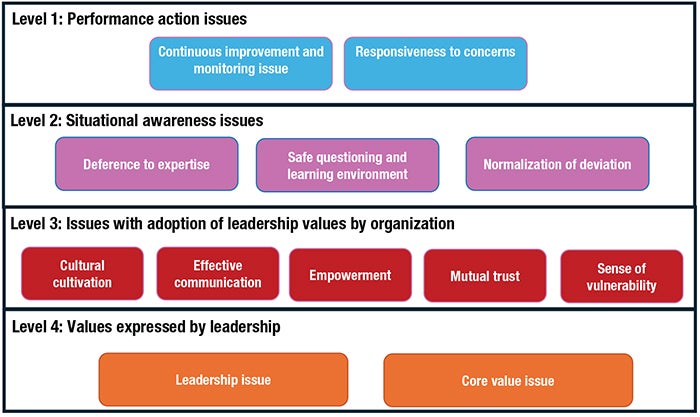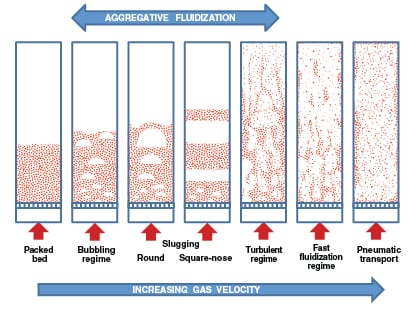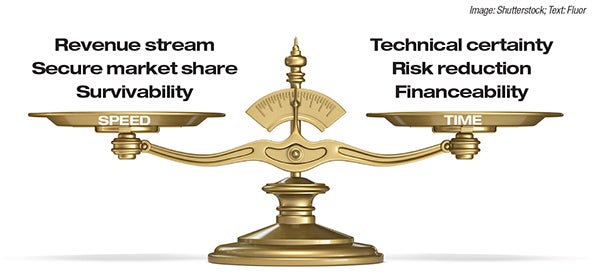
Gold Member Content
Current Issue

Webinars
More Webinars >
Speed of Thought in the Control Room: Turning Real-Time Data into Faster, Smarter Decisions
January 21, 2026
Unlocking AI Potential: The Role of a Modern Data Ecosystem
October 14, 2025

From Visualization to Action: Driving Results with AspenTech’s Real-Time Historian
September 24, 2025
Process Intensification Strategies for Biofuel Production
September 23, 2025