Isolation and purification of pure, active enantiomers is important in the development of active pharmaceutical ingredients and drug products. However, recovering the desired optical isomer from a racemic mixture means half of the chemical produced — the undesired isomer — will be wasted, unless it can be recycled in some manner. Now, researchers from the Tokyo University of Science (Japan; www.tus.ac.jp) have developed a system that, at least for one important class of compounds — chiral sulfoxides — is able to produce the desired isomer with high efficiency. This is done by a two-step process, as described in a recent issue of the Journal of Organic Chemistry.
In the first step (diagram), the desired isomer is separated from the racemic mixture in a high-performance liquid chromatography (HPLC) column containing a chiral stationary phase. The undesired enantiomer then passes through a photoreactor — a glass tube containing an immobilized photocatalyst (2,4,6-triphenylpyrrylium tetrafluoroborate) and transparent glass beads. Irradiation with blue light (402 nm) from a light-emitting diode (LED) causes a rapid photoracemization of the undesired isomer back into a racemic mixture, which can then be recycled back to the HPLC purification column. The cycle can be repeated until the yield of the desired compound is as high as desired.
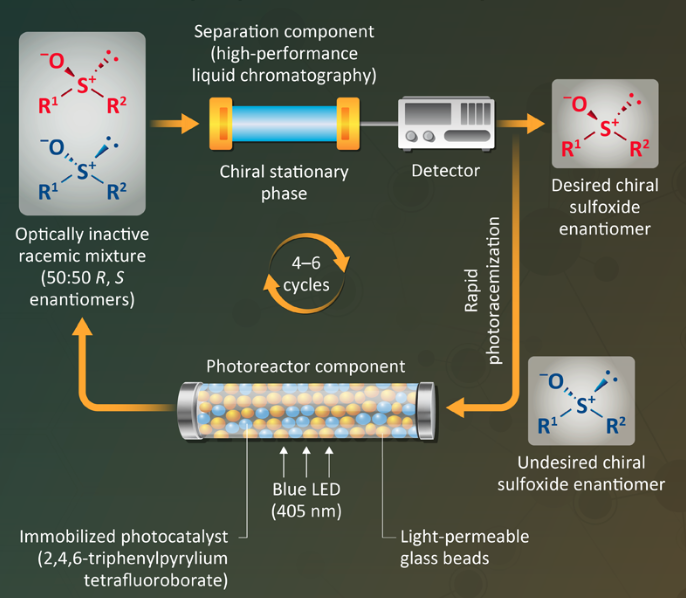
Source: Tokyo University of Science
The researchers demonstrated the technology for synthesizing four enantiometrically pure chiral alkyl aryl sulfoxides, achieving optical purities of 98–99%. High yields (>80%) for the desired compound are achieved after 4 to 6 cycles.