The ability to economically produce large scales of graphene will help to enable many high-performance applications, from smart packaging materials and medical devices to safer gas storage. A new process demonstrated at Southwest Research Institute (SwRI; San Antonio, Tex.; www.swri.org) has successfully produced graphene using CO2 gas, presenting a less-expensive feedstock alternative to traditional graphene production techniques, which usually rely on graphite or silicon carbide/hydrocarbon mixtures. The process involves bubbling CO2 through a bed of inexpensive liquefied alkali earth metals at lower temperatures than other methods (around 700ºC, versus 1,500ºC required for uncatalyzed CO2 splitting). “We specifically targeted a low-cost and highly available alkali earth metal for the initial testing. It has strong reduction properties, which enable the conversion of CO2 to carbon and a metal oxide. The process was developed to utilize scalable and lower-cost existing technologies in adjacent industries,” explains Michael Hartmann, manager of CCU-Process Development at SwRI.
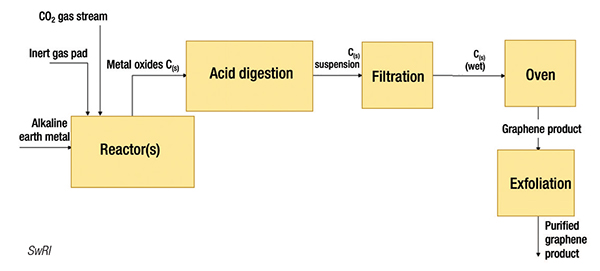
The reaction to produce graphene with alkali earth metal had previously been studied at the Harbin Institute of Technology in China, and now the SwRI team is building upon that foundation to provide a fully integrated plant design concept to demonstrate scalability. “Our research has uncovered novel reactor and mixing designs to increase yield and quality of the material produced,” says Hartmann. The main reaction steps require fairly simple equipment configurations, including continuously-stirred tank reactors and digestion vessels. So far, SwRI has produced gram quantities of graphene, and the team is working to expand to the kilogram scale and further improve efficiency and economics. “We are looking at ways to reduce the energy intensity of the process even further through investigating different eutectic mixtures and increase the yield and produce quality through reactor design, mixing efficiency and gas distribution,” says Hartmann.
An important part of scaling up the process is to minimize waste and feedstock costs. “There are key recycle steps to regenerate the alkali earth metals, along with the digestion chemicals. This reduces overall feedstock requirements, as well as reduces the process waste streams,” adds Hartmann. For every 200 grams of alkali earth metal the team can produce roughly 6 grams of graphene-containing material.
The purity of the CO2 feedstock also impacts process economics, but SwRI doesn’t see this as a concern going forward: “In the larger picture, as more CO2 pipelines and sequestration hubs come online, relatively pure CO2 streams will become available for use. This process is intended to tie into one of these sources to use a sequestered or otherwise low-cost waste stream and provide something of greater economic value,” he adds.