A team of researchers, led by professor Myoung-Jin Kim, from Korea Maritime and Ocean University (Busan, South Korea; www.kmou.ac.kr), has developed a process that recovers calcium-free magnesium sulfate from seawater desalination brine (SDB). The process, described in a recent issue of Desalination, takes advantage of the difference in solubility of MgSO4 and CaSO4 in ethanol.
In the process (diagram), alkali is first added to SDB to precipitate Mg(OH)2. The magnesium is concentrated by the addition of sulfuric acid. The eluate then undergoes a two-step addition of ethanol. First the ethanol dosage is adjusted so that only CaSO4 is precipitated. After the Ca2+ has been removed, ethanol is further added to precipitate the high-purity MgSO4. The magnesium recovery efficiency is 67%, producing 15.8 kg of MgSO4·7H2O from 1 ton of brine.
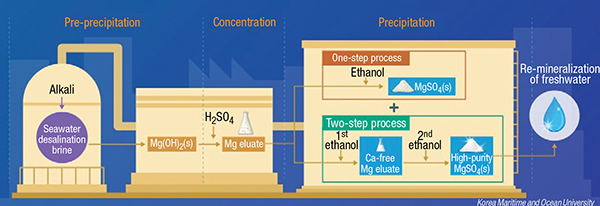
Because the purity of the recovered MgSO4 was up to 99.8%, it could be used to re-mineralize fresh water after the seawater desalination process. According to a cost assessment of the process, high-purity MgSO4 produced from seawater desalination brine is expected to be preferred over other MgSO4 products that are used in the pharmaceutical and food markets, which require high-purity and economic feasibility.
“Since we have already developed a sophisticated seawater desalination process to address the world’s water needs, why not couple it with the beneficial process of mineral extraction,” says Kim.