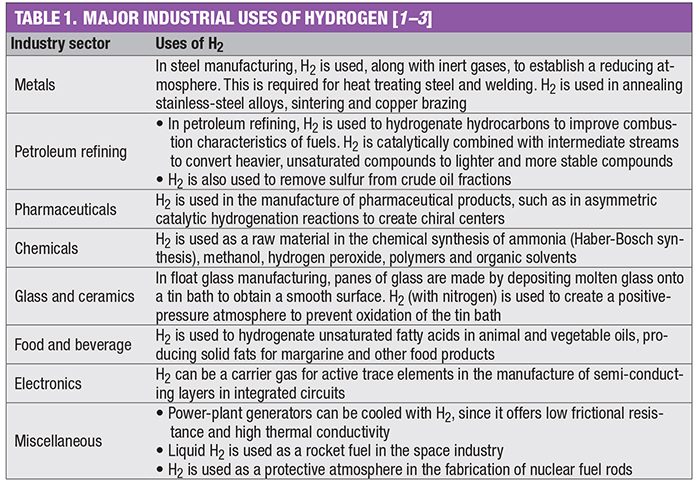
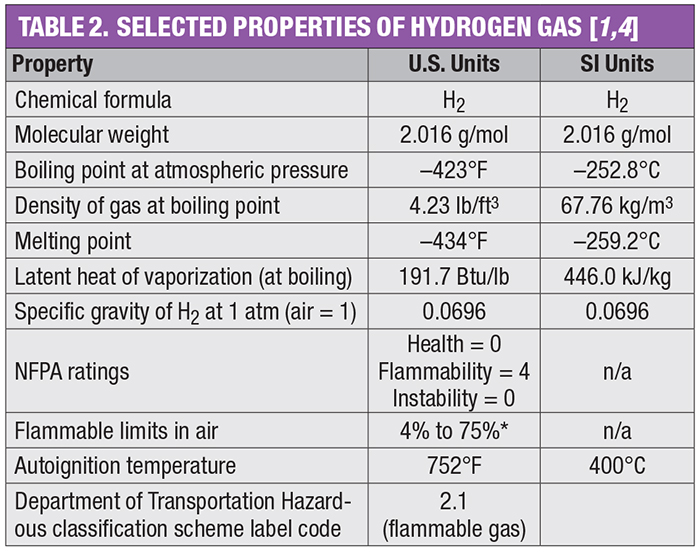
The use of molecular hydrogen is common across the chemical process industries (CPI). Annually, 70 million metric tons of H2 are produced worldwide. Most industrial H2 production currently occurs via steam reforming of methane, but production via water electrolysis is growing. This one-page reference provides information on H2 flame hazards and leak detection. Tables 1 and 2 outline major industrial uses of H2 and its key properties, respectively.
Hydrogen flammability
Although H2 is nontoxic, it is highly flammable and explosive. The National Fire Protection Association (NFPA; Quincy, Mass.; www.nfpa.org) rates H2 as a “4” on the flammability scale (the highest rating), because H2 is flammable when mixed with air, even in small amounts, and the minimum ignition energy (MIE) is small (0.019 mJ for a gas-air mixture). Hydrogen can also self-ignite without energy from an external source when it is leaking from a pipe at high pressure.
Hydrocarbon flames differ from H2 flames. AH2 flame emits low levels of infrared radiation and visible light, so it will not give off intense heat and light. Therefore, it cannot be easily detected by human senses. It is difficult to see a H2 flame even up close. Plant workers may see a shimmering, mirage-like area or possibly sparks, which are actually dust particles burning briefly in the flame [2].
H2 leak detection
Gas detection equipment can sense a H2 leak before it ignites, increasing the possibility of stopping a leak before it causes a fire or explosion. Catalytic bead (Pellistor) detectors sense H2 in combustible concentrations when it combines with oxygen to produce heat. This sensor usually consists of a matched pair of platinum wire-wound resistors, one of which is encased in a ceramic bead. The active catalytic bead is coated with a catalyst, while the reference catalytic bead remains untreated. The resistors are then enclosed behind a flame-proof sinter or porous filter. When the combustible gas comes in contact with the active catalytic bead surface, the gas is oxidized and heat is released, which changes the resistance of the wire. The reference (passive) bead maintains the same electrical resistance in clean air as the active bead, but does not catalyze combustible gas. Combustible gas concentrations are then determined by comparing the difference between the active and passive bead circuits. Limitations include the susceptibility to poisoning of the catalytic beads and their inability to signal a fault when they fail [2].
Hydrogen quickly floats upward and disperses, so detectors should be located close to and above spots where a leak might occur (just above a valve, for example).
Multi-spectrum NIR
If a flame ignites, multi-spectrum infrared (MIR) flame detection has become the preferred choice for detecting H2 flames in industrial settings. Some MIR detectors are designed specifically to detect the IR radiation from H2 flames. MIR flame detectors rely on a combination of IR filters and software analysis to detect flames and reduce the potential for false alarms.
References
1. Rivkin, C., Burgess, R. and Buttner, W., Hydrogen Technologies Safety Guide, National Renewable Energy Laboratory, NREL Technical Report, January 2015.
2. Hosch, M.J. and Paterson, A., Detection Systems for Reducing the Risk of Hydrogen Fires, Chem. Eng., April 2020, pp. 54–56.
3. Universal Industrial Gases Inc., Hydrogen Properties, Uses and Applications, web resources, www.uigi.com/hydrogen, accessed Nov. 2020.
4. Safety Data Sheet (SDS) for molecular hydrogen, 2018.