Following this approach to assessing health, safety and environmental risks can steer engineers to consider more inherently safe process options earlier in process synthesis
During process synthesis in the chemical process industries (CPI), engineers make decisions that determine the inherent health, safety and environmental (HSE) risk level of a process. This article proposes a simple approach for identifying and assessing the major HSE hazards during synthesis.
Understanding the risk level during synthesis enables the engineer to consider inherently safer designs (ISD) while developing the flow sheet. If risk levels are not considered during synthesis, higher risk processes will result, because decisions are hard to change once the flow sheet has been finalized. Most of the resistance to process design changes comes from trying to avoid the need to repeat expensive research and development work, as well as avoiding schedule delays.
To understand the value of ISD, consider the Bhopal, India incident where, in 1984, an accidental release of the chemical methyl isocyanate (MIC) occurred at a facility for manufacturing the insecticide Sevin. MIC, an intermediate in the production process for Sevin, is a highly toxic chemical. A National Research Council (Washington, D.C.; www.nationalacademies.org) study stated that more than 40 tons of MIC were released, killing close to 3,800 people and seriously injuring between 100,000 and 200,000 [1]. Had there been an inherently safer process at the Bhopal plant, the accident might have been prevented, or its severity greatly reduced.
What is process synthesis?
Process synthesis is the creation of a process for manufacturing a product that meets customer-based quality specifications, has an appropriate level of HSE risk, and meets business and economic requirements [2]. When complete, process synthesis will have defined the following:
• A block flow diagram of the process, showing all major process steps and flow streams
• Process operating conditions
• Material specifications for raw materials, catalysts, solvents, processing aids, packaging materials and so on
• Material and energy balances
• Required materials of construction
• Process design data, such as reaction kinetics and physical properties
• Scale-up criteria
• Major HSE hazards
How to think about HSE
HSE is a broad topic dealing with the safety of plant employees, of the physical plant, of the community and of the environment. Each of these constituencies is affected in its own way by a production process.
• Plant employees may be affected by chemical exposure, fire, explosion, noise and injuries
• The physical plant may be affected by fire, explosion and overpressure situations
• The community may be affected by accidental releases of substances, fires, explosions, noise, pollution, landfills and the disposal of hazardous chemicals
• The surrounding environment may be affected by planned emissions and discharges, toxic wastes, accidental releases and fugitive emissions
In the previous definition of process synthesis, the phrase “appropriate level of HSE risk” requires further explanation. Generally, risk is thought of as the following:
Risk = (Severity of Incident) × (Likelihood of Occurrence)
or
Risk = (Human and/or Economic Loss Potential) × (Probability of an Incident)
The definition of acceptable risk varies from company to company. Each organization must define what is appropriate for it, which is a difficult task at best. If the question was simply financial, such as weighing the cost of risk reduction against the monetary loss of an incident, it would be fairly straightforward to answer. The difficulty arises when one has to consider the loss of life or personal injury. What is a life or severe injury worth? What is an acceptable fatality rate? These are hard questions to answer.
In a recent article about COVID-19, Alex Berezow discussed the value of life [ 3]. Although he wrote the article from a geopolitical perspective, his remarks apply equally to HSE. He states there are three ways to view the value of life: morally/philosophically, economically and a blend of the two.
“Many people say that one cannot put a price tag on human life. This is true from a moral or philosophical perspective. … In the eyes of an economist, however, we are neither equal nor invaluable.
“How does one begin to put a price tag on a human life? A simple thought experiment demonstrates how easy it is. Pretend that you’re the mayor of a small town. There’s a dangerous intersection that results in one car crash fatality every year. A company approaches you with a solution: By redesigning and rebuilding the intersection, it can guarantee no more car crashes. The only problem is that the infrastructure project costs $100 million. Considering that your small town’s budget is far less than that, the city couldn’t afford it without a massive tax increase. You propose that tax increase to your citizens, who reject it overwhelmingly. The citizens have spoken: The lives of those who will be killed in accidents at the intersection are not worth $100 million.
“… For what it’s worth, various U.S. government agencies value a life between $9 million and $10 million.
“The key is to remember that we are talking about economic value rather than moral value. There is always space for a moral consideration in geopolitics, but it too must be considered along with other factors.”
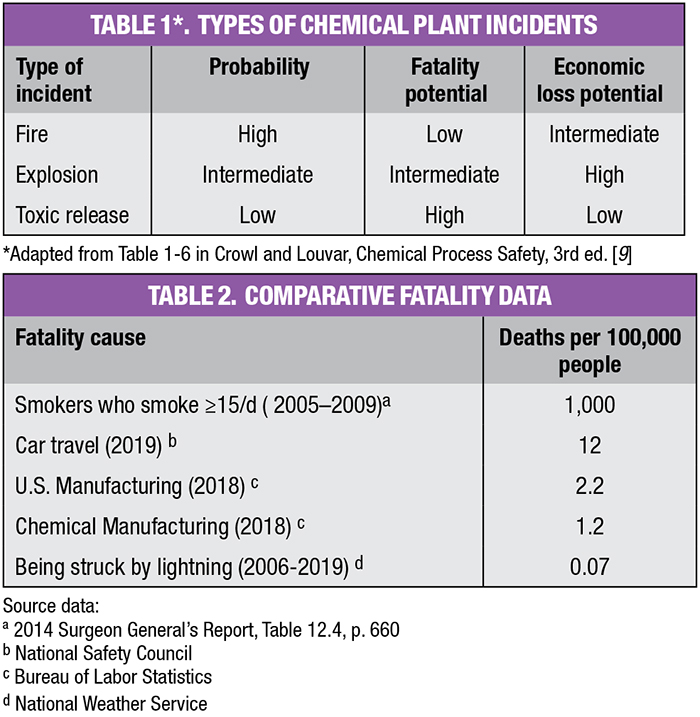
When thinking about risk, engineers should also consider the information shown in Tables 1 and 2, as well as their own company’s data and practice.
Methodology
The methodology presented here will help to find and assess HSE hazards during process synthesis. For hazards that could result in serious or catastrophic incidents, the engineer should consider changing the basic process to make it inherently safer. Because synthesis is the time when the process is being created, it is the ideal time to explore options for hazard elimination or mitigation. During synthesis, one need not work on routine safety issues, such as pressure relief. Later on, detailed studies, such as HAZOP (hazard and operability analyses), will identify these issues.
The steps in the methodology are the following:
• Identify the hazards
• Estimate the potential for loss
• Estimate the probability of having an incident
• Identify the major risks
• Consider inherently safer designs
Step 1: Identify process hazards. Begin by looking for toxic release, explosion and fire hazards, as follows:
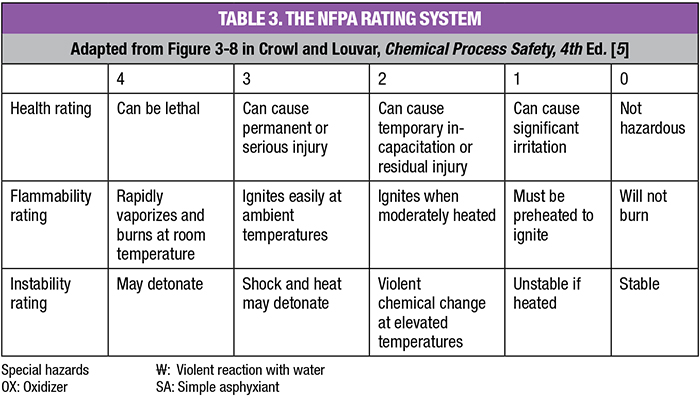
Hazardous materials. For each flow stream in the process, list the composition, materials and amounts of each stream. This should include intermediates and impurities. Consider feed streams, products, byproducts, recycle streams, purge, catalysts and solvents, as well as planned discharges to sewers, the atmosphere, landfills, recyclers and waste disposal firms. For each substance, determine whether it is hazardous; and if so, the degree of hazard it presents. One needs to consider health, flammability, instability and special hazards. An example of a material having a special hazard is MIC, which reacts violently with water. This is believed to be the initiating cause of the Bhopal incident mentioned previously. Usually, the National Fire Protection Association (NFPA; Quincy, Mass.; www.nfpa.org) or the U.S. Hazardous Materials Identification System (HMIS) ratings are sufficient sources of information to determine the hazard level of a chemical. Both systems have five rating levels. Table 3 explains the NFPA levels. HMIS is similar.
Step 2: Estimate loss potential. Once the hazards in the process have been identified, engineers should assess the potential for loss for each hazard. Because there are few design details at this point, these assessments will be “order-of-magnitude” quality. As such, they are only useful during synthesis. They do not take the place of the exacting safety studies done later during detailed design.
Referring back to Table 1, note that toxic releases and explosions have the greatest potential for fatalities and economic loss. To estimate each hazard’s potential for loss, place each into one of the following broad loss categories:
• Catastrophic — Those resulting in deaths or permanent disabilities, major community damage, or major plant damage
• Major — Those resulting in hospitalizations, significant community damage, or significant plant damage
• Moderate — Those resulting in injuries needing medical treatment, minor community damage, or moderate plant damage
• Minor — Those resulting in minor injuries, no community damage, or minor plant damage
When assessing the loss potential of materials, one can use NFPA or HMIS ratings to define the severity of health, flammability or instability hazards. If these are not available, use the Materials Safety Data Sheets (MSDS) for each substance or PubChem’s Compound Summary database [4]. If a material has an NFPA or HMIS rating of 4, assign its potential for loss as Catastrophic. A 3-rating should be assigned the loss category of Major, and so on. To illustrate, MIC, the toxic intermediate in the Bhopal Sevin process, has an NFPA health rating of 4. Thus, its loss potential would be Catastrophic. Its release was clearly that.
For process hazards, the guidelines are not as clear cut. The Marsh 100 Largest Losses Report provides perspective. Marsh, an insurance-brokerage and risk-management firm, regularly publishes global incident data. As this report only reports the largest global losses, it represents only the tip of the loss iceberg. Property losses follow a similar pattern to deaths and injuries. Crowl and Louvar suggest that for every 1 to 2 fatalities resulting from an incident, there are 10 to 20 serious injuries, 100 to 200 minor injuries, and 1,000 to 2,000 near misses [5].
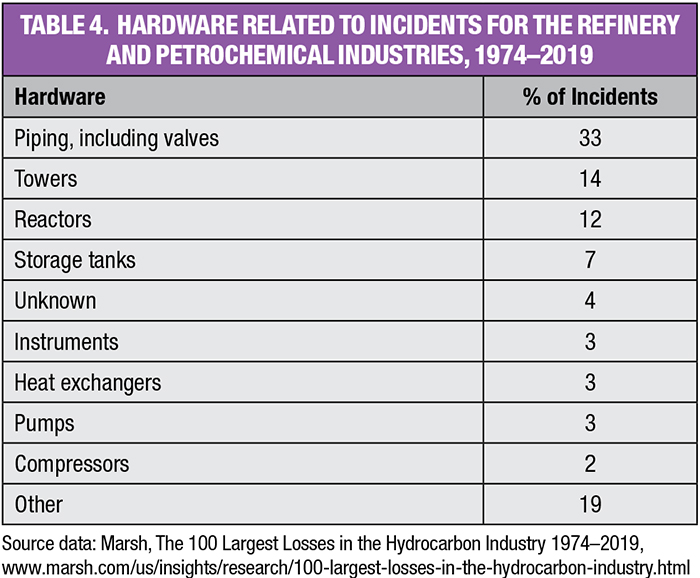
The March 2020 Marsh report covers the 46-year period from 1974 to 2019. It describes the 100 largest global hydrocarbon industry losses. Fifty eight of those came from the petrochemical and petroleum refinery segments. These resulted in losses totaling $23 billion, as well as the following:
• Losses averaged $500 million/yr
• The average cost of an incident was $397 million
• Three incidents (5%) resulted in toxic releases
• 39 (67%) began with an explosion
• 19 (33%) began with a fire
Table 4 summarizes the hardware and equipment associated with the 58 incidents from the petrochemical and refining sectors.
Step 3: Estimate the probability of an incident. As was the case with loss potential, it is impossible to make detailed estimates, so order-of-magnitude quality has to suffice at this point. Assign each hazard to one of four levels of probability, which are the following:
• Very Likely — occurring every 0–10 years
• Probable — occurring every 10–30 years
• Possible— occurring every 30–100 years
• Unlikely — occurring once every 100 years or more
As previously mentioned, larger losses occur less often; smaller losses are much more frequent. The synthesis engineer, when assigning one of the four probability levels to the hazards, should consult with experienced process safety experts and refer to company incident history.
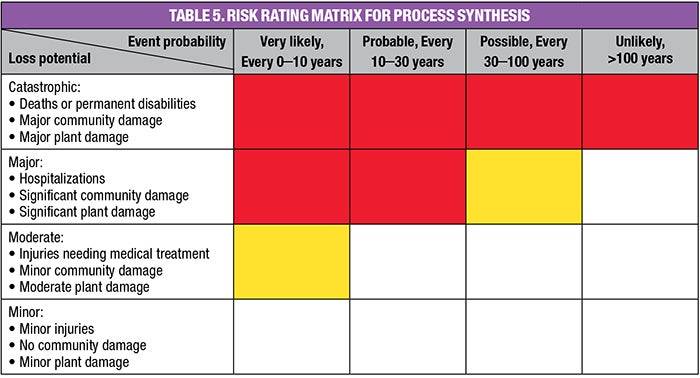
Step 4: Identifying major risks. Risk is the combination of loss potential and probability. When assessing risk during synthesis, use the Risk Rating Matrix (Table 5). First, enter the loss potential and probability estimates into the figure. If a material or other hazard falls into one of the red cells, it is important to work to eliminate, or greatly reduce, risk by applying ISD. Hazards falling into the yellow cells are borderline candidates for ISD.
Step 5: Consider inherently safer designs. The activities of hazard and risk identification lead directly to the question of what to do about high-risk hazards. In general, there are two options: use ISD principles or add safeguards to the process. Of the two, ISD is generally preferred because it focuses on the elimination or lessening of the hazard. It also delivers simpler, more straightforward designs. Kletz and Amyotte state, “Traditional plant designs try to reduce the risk by adding protective equipment and following safe methods of working. Inherently safer and friendlier plants remove or reduce the hazards” [6]. Protective equipment and safe practices will eventually result in an incident as equipment fails or people make mistakes.
Three pillars of ISD apply to process synthesis: substitution, minimization and attenuation. These pillars involve elimination of hazardous materials, use of much smaller quantities of them, and use of less hazardous operating conditions.
Substitution. This focuses on substituting non-hazardous or less-hazardous materials for hazardous ones. Consider the following:
- Using a reaction chain that doesn’t use hazardous materials (raw materials or catalysts) or that doesn’t produce hazardous intermediates or byproducts
- Pretreating the feed to eliminate impurities that react to form hazardous materials
- Using materials with higher flashpoints, boiling points and other properties that are well away from the operating conditions of the process
- Using different solvents, heat-transfer fluids or refrigerants to eliminate those that may be toxic or flammable
Minimization (intensification). The focus here is to minimize the amount of hazardous material in the system. Consider the following:
- Continuous or semi-batch systems, which usually hold smaller volumes of material compared to batch systems
- Changing reactor conditions — catalyst, temperature, pressure — to increase the reaction rate and make the reactor smaller
- Using a column sequence that minimizes the amount of hazardous material held in the process
- Using equipment having less holdup. A few examples are: divided-wall distillation columns, packed versus tray columns, thermosiphon versus kettle reboilers, plate versus shell-and-tube exchangers, and tubular versus pot reactors
Attenuation (moderation). Attenuation stresses the use of less hazardous operating conditions when handling hazardous materials and situations. Consider the following:
- Processing flammable materials well below their flash points and boiling points
- Handling explosive dusts as slurries
- Reducing the risks of a runaway reaction
- Using a smaller reactor (for example, a continuous versus batch reactor)
- Controlling the reaction rate via the order of chemical addition or by catalyst choices
- Adding inert materials to flammable mixtures
- Reducing process operating temperatures to eliminate the need for furnaces
Example
The following discussion about Bhopal and MIC illustrates substitution and minimization.
With an NFPA health rating of 4, MIC is very hazardous. As such, it would be placed in one of the red cells of the Risk Rating Matrix (Table 5). Thus, the reaction that produces it would be a prime candidate for ISD. As understood today, substitution is possible, because there are two accepted routes for making carbaryl (Sevin) [7]:
• The MIC route (used in Bhopal):
1. Methylamine + Phosgene ➔ MIC + 2 HCl
2. MIC + α-Naphthol ➔ Carbaryl
• The non-MIC route. This route uses the same feedstocks, but reacts them in a different order.
1. α-Naphthol + Phosgene ➔ Naphthol chloroformate + HCl
2. Naphthol chloroformate + Methylamine ➔ Carbaryl + HCl
Had the non-MIC route been used at the Bhopal, India facility, there would have been no incident — no deaths and no injuries. Even safer routes might be possible if something less hazardous could be substituted for phosgene, which has an NFPA Health rating of 4 [8].
There are also minimization options. Had the amount of MIC stored been small, far fewer people would have died or been injured. Recall, over 40 tons of MIC were released. It was this large release that caused the high number of deaths and injuries. Crowl and Louvar refer to a redesigned process that could reduce the MIC inventory to less than 20 lb [9]. In addition, the National Research Council in their report “The Use and Storage of Methyl Isocyanate (MIC) at Bayer CropScience,” refers to a process that produces gaseous MIC, which is consumed immediately [10].
Unaddressed risks/hazards
If significant hazards/risks exist that are not dealt with, they should be flagged so they will not be forgotten in the future. This helps ensure someone will design safeguards into the process to manage those risks and mitigate outcomes. For example, one of the senior chemical engineering design projects at the University of Cincinnati involved designing a greener process for making dimethyl carbonate [11]. The process uses a catalyst (methyl iodide) with an NFPA health rating of 3. The Safety and Hazard Section of PubChem’s Compound Summary for methyl iodide states that storing amounts greater than or equal to 7,500 lb “presents a potential for a catastrophic event …” [12]. This is the type of item one would flag, because storage systems are not dealt with during process synthesis.
Concluding remarks
The decisions made during synthesis set the HSE features of a process. Thus, it is imperative HSE be integrated into the process design. Especially with chemical processes, it is the only way HSE risk can be adequately dealt with. The approach in this article enables the synthesis engineer to identify and manage the major HSE hazards and risks in a process.
Edited by Scott Jenkins
References
- National Research Council, The Use and Storage of Methyl Isocyanate (MIC) at Bayer CropScience, Washington DC, 2012, p. 32.
- Brown, T.R., Cost Engineering: Integrating Technology and Economics, Chem. Eng., December 1, 2017, pp. 35–36.
- Berezow, Alex, Four Coronavirus Lessons That We Will (or Won’t) Learn, Geopolitical Futures, May 1, 2020.
- Cameo Chemicals MSDS, available at: https://cameochemicals.noaa.gov/chemical/1112.
- Crowl, D.A. and Louvar, J.F., “Chemical Process Safety,” 4th Edition, Prentice Hall, 2019.
- Kletz, T.A, and Amyotte, P., “Process Plants: A Handbook for Inherently Safer Design,” 2nd Edition, CRC Press, 2010.
- Crowl, D.A. and Louvar, J.F., “Chemical Process Safety,” 2nd Edition, Prentice Hall, 2002, p. 26.
- Cameo Chemicals MSDS, https://cameochemicals.noaa.gov/chemical/4228.
- Crowl, D.A. and Louvar, J.F., “Chemical Process Safety,” 3rd Edition, Prentice Hall, 2011.
- National Research Council, The Use and Storage of Methyl Isocyanate (MIC) at Bayer CropScience, Washington, D.C., 2012, p. 114.
- An unpublished report by: Ballachino, K., Barr, C., Brown, N., Gunther, A., and Vennefron, Evaluation of the Production of Dimethyl Carbonate (DMC) through a Catalytic Reaction, March 2020.
- PubChem Compound Summary, available at: https://pubchem.ncbi.nlm.nih.gov/compound/6328#section=NFPA-Hazard-Classification.
Author
 Thane Brown (Email: [email protected]) worked for more than 36 years for Procter & Gamble in a variety of engineering and manufacturing roles, primarily in the food-and-beverage business and in health, safety and environmental engineering. In his last position there, Brown was director of North American engineering. After retiring, he taught engineering economics at the University of Cincinnati, and plant design at the University of Dayton. Brown is presently a member of the Chemical Engineering Advisory Committees at the University of Dayton, at Miami University (Oxford, Ohio), at the University of Louisville and at the University of Cincinnati. He also works as a SCORE counselor, providing free assistance to small businesses in the Cincinnati area. Brown authored the book “Engineering Economics and Economic Design for Process Engineers,” as well as a number of articles on engineering economics, batch pressure filtration and heat transfer. He is a registered professional engineer in Ohio (inactive), and holds a B.S.Ch.E. from Oregon State University.
Thane Brown (Email: [email protected]) worked for more than 36 years for Procter & Gamble in a variety of engineering and manufacturing roles, primarily in the food-and-beverage business and in health, safety and environmental engineering. In his last position there, Brown was director of North American engineering. After retiring, he taught engineering economics at the University of Cincinnati, and plant design at the University of Dayton. Brown is presently a member of the Chemical Engineering Advisory Committees at the University of Dayton, at Miami University (Oxford, Ohio), at the University of Louisville and at the University of Cincinnati. He also works as a SCORE counselor, providing free assistance to small businesses in the Cincinnati area. Brown authored the book “Engineering Economics and Economic Design for Process Engineers,” as well as a number of articles on engineering economics, batch pressure filtration and heat transfer. He is a registered professional engineer in Ohio (inactive), and holds a B.S.Ch.E. from Oregon State University.