By Intratec Solutions
Maleic anhydride (MAN) is a multifunctional chemical intermediate with applications in several fields of the chemical process industries (CPI). Its major end use is as feedstock in the production of unsaturated polyester resins (UPR). In addition, maleic anhydride can be used as a raw material in the production of 1,4-butanediol (BDO), gamma-butyrolactone (GBL) and tetrahydrofuran (THF). BDO has been one of the world’s fastest growing chemicals in recent years.
The process
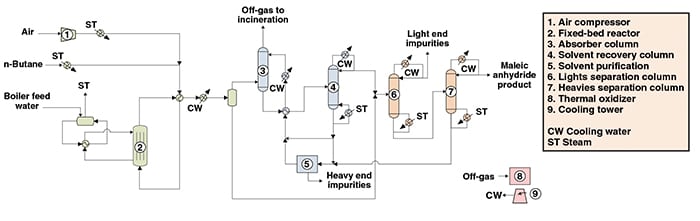
The following paragraphs, along with Figure 1, describe a process for producing MAN from n -butane. The process described is a fixed-bed technology similar to the one owned by Huntsman Corp. (Danbury, Conn.; www.huntsman.com).
Reaction. Fresh butane is vaporized and mixed with air before being fed to the reactor. The oxidation reactor consists of a fixed-bed, multi-tubular reactor containing a vanadium phosphorus-oxide catalyst supported on silica.
The heat generated in the highly exothermic reaction is removed by a molten salt circulated through the jacket that surrounds the reactor tubes. The molten salt is then passed through an external cooler and cooled by boiler feedwater, producing steam.
The reactor exit-gas stream is mainly comprised of maleic anhydride, water vapor, carbon oxides, oxygen, nitrogen and unconverted butane. This stream is then partially condensed. The condensate stream is sent to the purification area, while the gas stream is sent to an absorber column for recovery of the remaining MAN.
MAN recovery. MAN that was not condensed is recovered by absorption with a solvent. The residual gas (containing mainly nitrogen, CO, CO2 and unreacted butane) is removed at the top of the column. This stream is sent to a thermal oxidizer for incineration at offsite facilities.
The recovered stream from the bottom of the absorber column is sent to a solvent recovery column, in which MAN is recovered as the distillate. The MAN recovered in this step is mixed with the condensate from the reaction area and then sent to two purification steps for obtaining pure maleic anhydride.
The solvent, recovered from the bottom of the solvent-recovery column, is returned to the absorption column. A small portion of the solvent stream is sent to a solvent-purification step for removal of impurities.
Purification. The purification area consists of two distillation columns for the removal of light-end impurities and heavy-end impurities. The final product from purification is MAN with purity greater than 99.8 wt.%.
Economic performance
The total capital investment estimated to construct such a plant producing 60,000 metric ton (m.t.) per year of maleic anhydride in the U.S. is about $150 million. The capital investment presented includes the total fixed capital, working capital and additional capital requirements. The production cost is estimated at about $830/m.t. of MAN. Other assumptions made are:
- Period of the analysis was the first quarter of 2015.
- The plant includes tanks providing a total storage capacity of 20 days for n -butane raw material.
Global perspective
Maleic anhydride was first commercially produced in the 1930s via benzene oxidation. However, the increase in the price of benzene, as well as the recognition of benzene as a hazardous compound, boosted research toward alternative pathways for MAN production. Currently, MAN is mostly produced using n -butane as the feedstock. Figure 2 illustrates alternative pathways for the production of maleic anhydride.
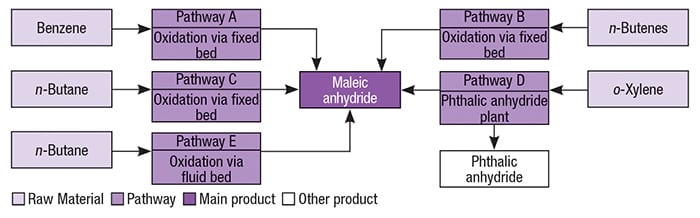
Figure 1. The process shown for maleic anhydride from n-butane via fixed-bed technology is similar to Huntsman Corp.’s
Editor’s Note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.