Site commissioning is underway at a facility in Nebraska that uses plasma pyrolysis to generate hydrogen gas and carbon black from natural gas without any local carbon dioxide emissions. The developer, Monolith Materials (Lincoln, Neb.; www.monolithmaterials.com), scaled up the process at the site after operating a pilot plant from 2014 to 2018.
 In a second phase of the project, currently in the front-end engineering and design (FEED) stage, the pyrolysis-derived H2 will be combined with N2 from the atmosphere to make ammonia for agricultural fertilizer via a Haber-Bosch process.
In a second phase of the project, currently in the front-end engineering and design (FEED) stage, the pyrolysis-derived H2 will be combined with N2 from the atmosphere to make ammonia for agricultural fertilizer via a Haber-Bosch process.
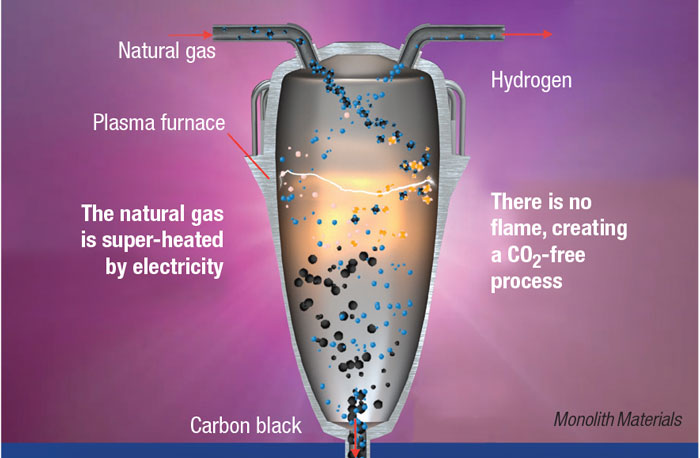
In Monolith’s proprietary methane-pyrolysis process, natural gas is fed into a reactor along with other process gases, where it is heated to between 1,500 and 2,000°C by electric plasma. The heat splits the CH4 into H2 and solid carbon in the absence of O2. “The reaction conditions, with close attention on the fluid dynamics of the gases and the aerodynamics of the solids, allow us to finely control the particle morphology of the carbon black at the nanoscale, and also to avoid unwanted side reactions,” explains Rob Hanson, co-founder and CEO of Monolith.
To achieve H2 production at commercial scale, the company met the challenge of running the plasma pyrolysis reactor reliably for long periods at the high temperatures required for methane splitting. The carbon black produced by the reaction is sold into the vehicle tire market and used for battery materials, while the H2 could be used for ammonia or in the petroleum-refining industry.
The environmental advantages of the electric plasma-pyrolysis process are significant. Production of H2 in this way uses only one-seventh the power required to produce H2 via conventional electrolysis of water, and purified water is not required. And if the electricity to generate the plasma comes from renewable sources, it would allow fully emissions-free production of H2. Natural gas for the process could also be sourced from landfill biogas, Hanson notes.
Full commercial-scale production of what is termed “turquoise ammonia” (NH3 produced using H2 from methane pyrolysis) at the facility is expected in 2024.