A process that converts process gases, generated during the production of metallurgical coke, into marketable chemicals is being developed in a pilot plant installed on the works site of thyssenKrupp Steel Europe AG (www.thyssenkrupp-steel-europe.com) in Duisburg, Germany. The process is being developed in a collaborative project by the Schwelgern coke plant (KBS), plant-engineering company thyssenKrupp Industrial Solutions AG (Essen; www.thyssenkrupp-industrial-solutions.com) and Berlin Technical University (TU Berlin; all Germany; www.tu-berlin.de).
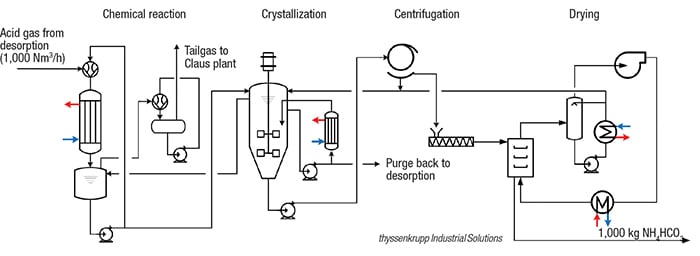
In the conventional treatment of coke-oven gas (COG), H2S and NH3 are scrubbed from COG with aqueous ammonia solutions (de-acidified water, stripped water) by the CyclaSulf process. The rich absorption solution (enriched water) is pumped to the regeneration, H2S/NH3-desorption column. The head product of the H2S/NH3-desorption is acid gas, which is rich with H2O, NH3 (20–30 vol.%), CO2 (15–25 vol.%) and H2S. The rate of H2O, NH3 and CO2 in acid gas is nearly equimolar, which makes it possible to synthesize crystalline ammonium bicarbonate.
In the new, patented process (flowsheet), the acid gas from the CyclaSulf process is first compressed (by acid-gas-condensate jet flow) in a jet ejector, where a spontaneous condensation of H2O and NH3 takes place. The gas-liquid mixture flows directly from the ejector through a falling film apparatus, in which selective conditions accelerate the chemical absorption of CO2 by NH3. The condensate flows to a vessel, and the gas (mostly CO2, H2S and HCN) is directed to the Claus plant or the sulfuric-acid plant. In the collecting vessel, the absorbed CO2 reacts further to HCO3 –, and the NH3 to NH4 +. The condensate in the collecting vessel, which is produced under specific reaction conditions, is an under-saturated mother liquor of NH4HCO3. As the mother liquor is fed to the crystallizer, a decrease in temperature causes the NH4HCO3 to crystallize and precipitate from the solution. The suspension is then centrifuged and the crystalline NH4HCO3 dried to achieve the final product quality.
With the pilot plant, 95% of the ammonia contained in the COG can be utilized, producing 15 kg/h of solids from 15 Nm3 of acid gas and 2 Nm3 of CO2. A commercial plant — for example a medium-sized coke plant with a capacity of 150,000 Nm3/h COG and a concentration of 6 gr/Nm3 ammonia in COG — could produce 4.2 ton/h of NH4HCO3, in addition to the other byproducts produced (tar, COG, sulfur and benzene, toluene and xylenes).
The end products can be put to a range of uses, such as nitrogen fertilizers, propellants and foaming agents for plastics or porous ceramics, and also in the food industry (baking soda). Although the process economics have to be evaluated individually for each coke plant, some samples of byproduct plants that have been economically analyzed have shown a return on investment after 3–5 years, say the companies.