Polystyrene (PS) is among the more difficult-to-recycle fractions of plastic waste, but could be a source of valuable aromatic petrochemicals, such as toluene. Prior approaches to convert PS to toluene have exhibited low yields and a range of products. Now, a group led by researchers at the Nanjing Forestry University (Nanjing, China; eng.njfu.edu.cn) and Tsinghua University (Beijing, China; www.tsinghua.edu.cn) has developed a process for converting PS waste to toluene in high yields and with excellent selectivity. In addition, a techno-economic analysis of the process indicates it could reduce the carbon footprint of the process and produce toluene at costs substantially lower than the current industry benchmark for petroleum-based toluene.
To carry out the conversion, the research team used a two-stage, fixed-bed reactor that was previously developed by the group, and a catalyst specially designed with single-atom ruthenium active sites (Figure). In the first stage of the process, polystyrene waste is thermally depolymerized under pyrolysis conditions into aromatic intermediates. Then, the hot vapor flows into a second stage of the process, where a catalyzed hydrogenolysis process selectively converts the gas-phase aromatic intermediates and hydrogen gas into toluene.
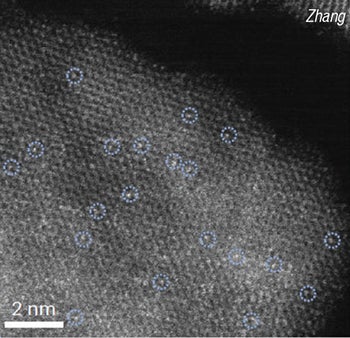
The single-atom ruthenium, cobalt oxide catalyst (RuSA/Co3O4) “was designed to selectively cleave C–C bonds in key intermediates, steering the reaction towards the formation of toluene,” the researchers write. “During the thermal decomposition of the oxide precursor, defects generated in the Co3O4 lattice can effectively trap isolated Ru atoms, leading to the formation of Ru-Co3O4 single-atom catalysts,” says Zedong Zhang, one of the lead researchers on the study, now a postdoctoral fellow at Northwestern University (Evanston, Ill.; www.northwestern.edu). The research was published in Nature Nanotechnology in December 2025.
The RuSA/Co3O4 catalyst demonstrated a toluene selectivity of 99%, with a yield of 83.5 wt.% and a formation rate of 1,320 mmol per gram of catalyst per hour. “Such selectivity streamlines product separation and improves energy efficiency, facilitating scalable plastic waste valorization,” Zhang says.
The researchers also reported that the RuSA/Co3O4 catalyst maintained structural integrity and performance over 100 h of continuous operation. The researchers plan to work on optimizing the system with an eye toward industrial translation, and generalizing the process to feedstocks other than polystyrene.