Polylactic acid (PLA, polylactide; Figure 1) is a thermoplastic aliphatic polyester that is derived from a number of renewable starting materials, such as starch and sugarcane, via bacterial fermentation. PLA is semicrystalline or amorphous, rigid, resilient and an ultraviolet-light-resistant material, with similar characteristics to common petrochemical industry plastics, such as polyethylene, polypropylene and polystyrene. PLA is used in plastic films, bottles and biodegradable medical devices. It figures among the most highly consumed bio-based plastics worldwide.
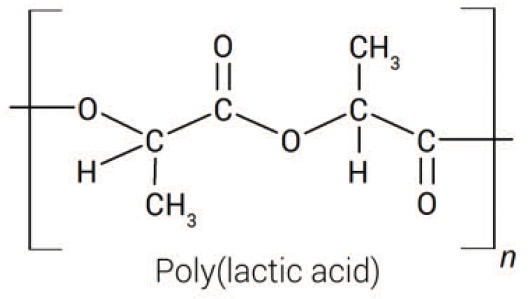
Figure 1. PLA is an aliphatic polyester derived from renewable starting materials
PLA rapidly absorbs moisture from the atmosphere and is also temperature-sensitive (storage temperatures should be below 122ºF). In order to preserve PLA properties and characteristics, exposure to ambient (undried) air should be minimized. PLA is usually delivered in moisture-resistant containers, including foil-lined boxes, which can prevent moisture regain during shipping and storage. PLA is mainly used in packaging, textiles, nonwoven fabrics, electronics, automotive parts, medical devices and cutlery. Packaging is the largest application market for PLA, and will likely grow as new heat-resistant PLA blends are developed.
Polylactic acid process
The production of PLA from corn (Figure 2) involves three major sections: (1) Starch hydrolysis; (2) Glucose fermentation; and (3) Lactic acid polymerization.
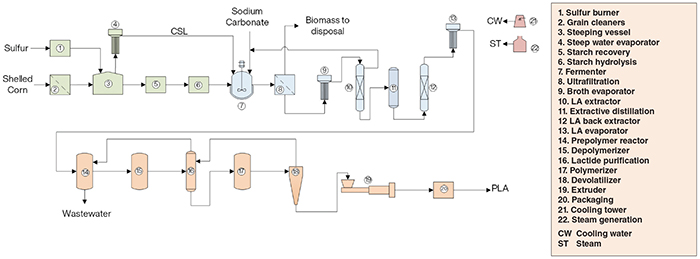
Figure 2. The diagram shows the production process for PLA, starting from corn
Starch hydrolysis. Shelled corn is fed to grain cleaners, then sent to steeping vessels, where it is soaked in a dilute solution of sulfur dioxide, produced in a sulfur burner. The solid matter slurry obtained is submitted to a sequence of unit operations to recover cornstarch, while the steep water is concentrated in evaporators and sent as corn steep liquor (CSL) to the downstream process stages. The starch is then mixed with enzymes and sodium carbonate in the hydrolysis area to convert it to glucose, which is sent to the fermentation stage.
Glucose fermentation. The fermentation is carried out in an agitated jacketed fermenter, fed with glucose, CSL and sodium carbonate. The fermentation broth is sent to an ultrafiltration step to remove cell debris, concentrated in an evaporator, and then to an extraction column to extract the lactic acid (LA) in an organic phase, which is routed to a distillation column to separate the extractant solvent from the LA. The LA-rich stream is then back-extracted with hot water in an extractor train, generating a LA-lean organic phase, which is recycled to the first extraction stage, and an aqueous phase rich in LA that is concentrated in an evaporator and sent to the polymerization stage.
Lactic acid polymerization. The concentrated LA stream is fed to a prepolymer reactor, where it forms low-molecular-weight PLA and water, which is removed and disposed. The low-molecular-weight PLA is sent to the depolymerizer and broken into lactide molecules (the cyclic lactone di-ester derived from lactic acid), which are purified in a drying column and in a meso-lactide removal column. Lactide is fed to a polymerization reactor, forming PLA, which is sent to a devolatilizer to remove unreacted lactide. The PLA is routed to an extrusion step and then packed in bags for storage.
Production pathways
This biodegradable polyester can be synthesized via two major routes. The main production route for high-molecular-weight PLA is based on an initial polycondensation of lactic acid, followed by a depolymerization at reduced pressure and a ring-opening polymerization step. The other route is based on a direct polymerization of lactic acid by polycondensation. Most companies employ the indirect route, since the production of high-molecular-weight polymers is difficult via direct polymerization.
Edited by Scott Jenkins
Editor’s note: Content for this column was originally developed by Intratec Solutions LLC (Houston; www.intratec.us) and is edited by Chemical Engineering. The analyses presented are based on publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing the analyses can be found, along with terms of use, at www.intratec.us/che.