This column is based on “Polyvinyl Chloride Production from VCM (Suspension Polymerization) – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/polyvinyl-chloride-production-cost.
Polyvinyl chloride (PVC) is the third most commonly produced synthetic polymer, after polyethylene and poly-propylene. PVC is a white, brittle solid and it comes in two basic forms: rigid (RPVC) and flexible. It can be com-pounded for a wide range of properties, which allows PVC to be used in a broad range of applications.
This commodity thermoplastic can be manufactured through different polymerization processes, however, most commercial PVC production is based on a suspension polymerization processes. To a lesser extent, PVC is produced at industrial scales via emulsion polymerization and bulk (mass) polymerization.
The process
The suspension process for PVC production comprises three major sections: (1) polymerization; (2) vinyl chloride monomer (VCM) recovery; and (3) drying (Figure 1).
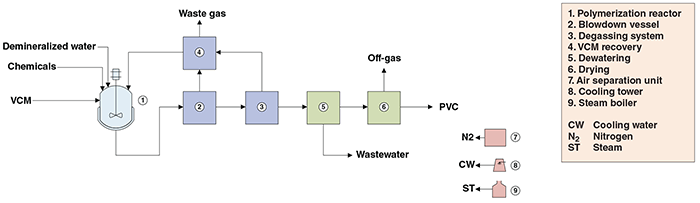
Figure 1. The diagram shows polyvinyl chloride production from VCM (suspension polymerization)
Polymerization. The VCM feed stream, along with hot demineralized water and chemicals (polymerization initiators, dispersing agents and additives) are supplied to a stirred reactor. The reaction occurs substantially in the liquid phase, in VCM droplets dispersed in water, which is used as diluent. After the initiation of VCM polymerization, the heat from the highly exothermic reaction is removed by means of a cooling system, for isothermal temperature control. The molecular weight of the polymer produced is defined by the length of the resulting polymer, which depends on the polymerization temperature. As the polymer is formed, it precipitates out of the reaction medium, floating in the liquid monomer phase. The polymerization is interrupted when the conversion of VCM to PVC reaches about 90%.
VCM recovery. The polymer slurry is fed to a blowdown vessel, operated at a lower pressure than the reactor. Most of the unreacted VCM flashes off. The polymer slurry is pumped to a pre-degassing vessel and a sieve-tray degassing column, where remaining VCM is removed from the polymer suspension. The VCM-rich off-gases are compressed and condensed in liquid-ring compressors and fed to a vessel, in such a way that non-condensed light contaminants are separated from the liquid VCM and released as waste gas. The VCM obtained is recycled to the polymerization reactor.
Drying. The slurry is passed through a solid-bowl decantation centrifuge to obtain a wet PVC cake with residual moisture, which is conveyed to a drying step. The drying stage is a combination of a flash dryer with a cyclone dryer, where the wet PVC cake is dried until its moisture content falls within the 0.2–0.3 wt.% range. The dry PVC powder from the drying stage is then packed for storage.
Production pathways
PVC production from VCM can proceed via several different manufacturing routes, each related to different sources of this monomer. In this context, the most typical PVC production routes are based on vinyl chloride produced by reacting ethylene with chlorine (Figure 2).
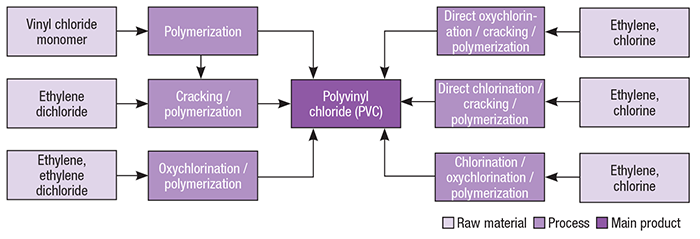
Figure 2. PVC production pathways are based on the sources of the monomer
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce PVC was about $850 per ton of PVC in the second quarter of 2017. The analysis was based on a plant constructed in the U.S. with the capacity to produce 400,000 metric tons per year of PVC.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.