Researchers from the College of Chemical Engineering, Nanjing Tech University (Nanjing, China; www.njtech.edu.cn) have developed a new strategy for forming lithium ion-sieve membranes to achieve an efficient recovery of lithium ions from brine or seawater.
Spinel lithium manganese oxide ion-sieves have been considered the most promising adsorbents to extract Li + from brines or seawater. The researchers have reported a lithium ion-sieve which was loaded onto tubular alpha-Al2O3 ceramic substrates by dipping crystallization and post-calcination (diagram).
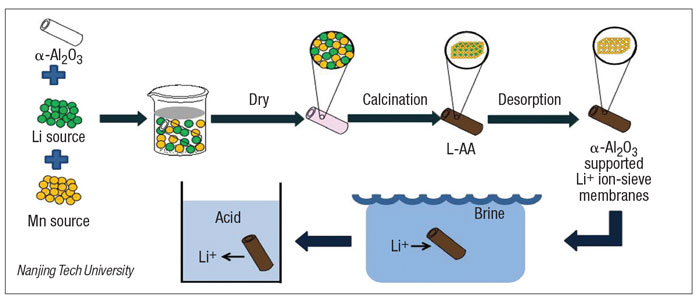
The lithium manganese oxide (Li4Mn5O12) was first synthesized onto tubular alpha-Al2O3 ceramic substrates as the ion-sieve precursor and the corresponding lithium ion-sieve was obtained after acid pickling. The lithium manganese oxide could be uniformly loaded not only onto the surface of alpha-Al2O3 substrates but also inside the pores. The equilibrium adsorption capacity of the lithium ion-sieve was 22.9 mg/g. The adsorption balance was reached after 12 hours. After five adsorption cycles, the adsorption capacity of the lithium ion-sieve was 60.88% of the initial capacity.
For the dynamic adsorption-desorption process, the lithium ion-sieve exhibited excellent adsorption performance for Li+, with the Li+ adsorption capacity of 9.74 mg/g and a manganese ion (Mn2+) dissolution loss rate of 0.99%. After three dynamic adsorption-desorption cycles, 80% of the initial dynamic adsorption capacity was still maintained.