Increasingly, wastewater treatment plants must address odors due to volatile contaminants. This guide provides an overview of odor causes and possible remedial actions
Processes and applications that emit odor-causing air contaminants are common across many sectors of the chemical process industries (CPI). In many cases, including wastewater treatment plants (WWTPs), paper mills, asphalt refineries and other operations, large volumes of odor-causing compounds are generated, and residents of the areas surrounding the facility can be exposed.
In the past, these odors were less of an issue because most industrial facilities tended to be located at considerable distances from large residential areas. In more recent times, however, there has been an increase in the number of industrial operations that are located within or near small towns and even large cities. When local residents constantly smell these industrial processes, unhappy neighborhoods are generally the result.
The collective voice of the public is now often strong, and has resulted in new regulations regarding odors and odor control at industrial facilities. Many industries can no longer produce odors without facing the consequences of either constant complaints, or fines from federal, state and local authorities. In addition to being a nuisance, these foul odors can pose a health and safety concern for the community, especially at high concentrations.
Furthermore, increases in population and associated industrial growth have driven an increase in the amount of waste requiring processing, and a subsequent increase in the odor problem.
This article provides information about common odor-causing compounds and about possible methods for controlling those odors, with a particular focus on WWTPs.
Odors and health hazards
In general, odors are subjective, so that a particular type of odor may bother one person while having little or no impact on another. The odor threshold is the minimum concentration of an odorous compound that is necessary to be detected by 50% of the population [1].
In principle, all chemicals can theoretically be toxic at high enough concentrations. For instance, water is not normally considered toxic. However, drinking an excessive amount of water under certain circumstances can create a condition called hyponatremia [2]. A similar situation applies to the concentration of odorous gases. At low concentrations (parts per billion; ppb), some gases can be detected by most humans. However, those gases are not toxic until the concentrations reach low parts-per-million (ppm) levels. Unfortunately, many industrial processes produce odorous gases at concentrations that are both a nuisance and that can present real health hazards.
Ammonia, hydrogen sulfide, mercaptans and volatile organic compounds (VOCs) are examples of odorous gases that may be produced in the processes of several types of industrial facilities, including (but not limited to) WWTPs, asphalt refineries, hot mix plants, landfills, farms and others.
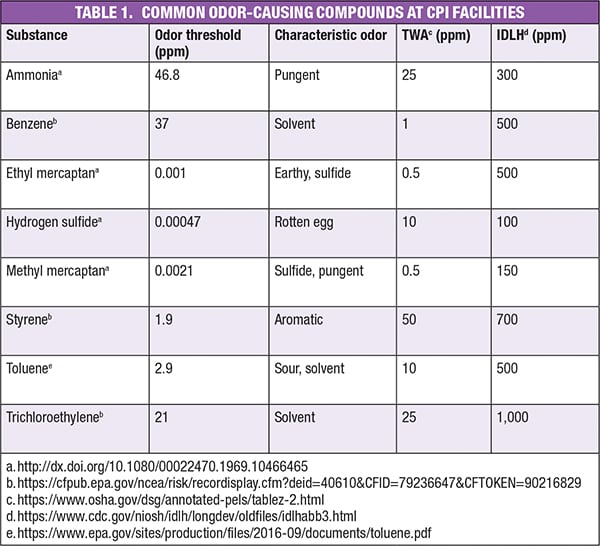
Table 1 lists several common odorous gases associated with these industry sectors. The table includes odor thresholds, characteristic odor, time-weight average (TWA) and immediately dangerous to life or health (IDLH) levels for each gas. The TWA represents the concentration of a given chemical that will not cause adverse effects when the exposure reaches eight hours per day. In most cases, the TWA is higher than the odor threshold; meaning that you can smell the odor before there is any potential hazard. The IDLH is the level at which a chemical can cause death or permanent adverse health effects. Notice that the IDLH is a higher concentration than the odor threshold for most of these chemicals. However, in the case of hydrogen sulfide, the human nose can become desensitized to the gas at concentrations lower than the IDLH. This means that humans may not smell the hydrogen sulfide at levels where it can cause severe harm or death.
The following descriptions cover several of the most common odorous gases found in the CPI.
Ammonia. Ammonia is a colorless gas that is highly irritating with a pungent odor at room temperatures. It is soluble in water, corrosive, and has alkaline properties. It can cause hoarseness, violent coughing, painful breathing, impaired vision, dyspnea and cyanosis when exposed to high levels [3].
Hydrogen sulfide. Hydrogen sulfide is a highly flammable, extremely hazardous gas that has a characteristic “rotten egg” smell. Hydrogen sulfide has an odor threshold of 0.00047 ppm, meaning that 50% of a human panel can smell it at that low of a concentration [4]. At a concentration of around 150 ppm, olfactory nerves can become paralyzed, resulting in humans having the sense that the smell disappears. Hydrogen sulfide is lethal at 700–1,000 ppm within minutes [5–7]. A complication with hydrogen sulfide is that your sense of smell disappears at only 150 ppm. Therefore, one could be exposed to higher levels of hydrogen sulfide and not know that it is present. It is soluble in water, corrosive and toxic to both humans and the environment.
Mercaptans. Mercaptans, such as ethyl mercaptan, are compounds that contain a carbon-bonded sulfhydryl group (–C–SH or R–SH, where R represents an alkane, alkene or other carbon-containing group of atoms). Mercaptans are flammable, have strong odors, and can be detected by the human nose at concentrations in the parts per billion range. Ethyl mercaptan is intentionally added to propane at low concentrations in order to help warn of a potential gas leak. At higher concentrations, according to OSHA (U.S. Occupational Safety and Health Administration; Washington, D.C.; www.osha.gov) regulations, ethyl mercaptan is classified as an acute toxic material for oral ingestion and inhalation, as well as for skin sensitization [8].
VOCs. VOCs, such as styrene, toluene and trichloroethylene, are organic chemicals that have high vapor pressures at room temperature. Their high vapor pressure allows them to either evaporate or sublime, respectively, from a liquid or solid to a gas. Hazardous VOCs present safety concerns, because they can be flammable, can cause cancer and chronic health effects, and may be lethal at certain concentrations. For example, toluene is a VOC found in many industrial applications, including both wastewater treatment plants and asphalt refineries. It has a high odor threshold of 2.9 ppm, which indicates that the presence of the odor may not be a good judge of safety for this chemical. Toluene poses a safety concern, as it presents acute toxicity hazards for oral ingestion. It is also a skin and eye irritant, and it may cause damage to organs through prolonged or repeated exposure. With an odor similar to paint thinner, it is an aromatic hydrocarbon, and is insoluble in water [9].
Odors at WWTPs
Wastewater treatment plants (WWTPs) generate odors during many of their normal processes, including collection, treatment and disposal (Figure 1). Incoming waste includes both household and industrial sources, which both contain a number of different types of odor-causing compounds. Industrial sources often cause concern because the waste can contain a number of toxic chemicals. Other odor-causing chemicals do not directly come into a WWTP from households or industrial sources. They are generated by anaerobic biological activity that consume the organic material found in the wastewater.

Figure 1. Wastewater treatment plants can be sources of odor-causing gases, such as hydrogen sulfide, ammonia, mercaptans and others
One of the most common odorous gases found in WWTP is hydrogen sulfide (H2S). It is produced in wastewater by the reduction of sulfate to hydrogen sulfide gas by bacteria. At a pH above nine, the hydrogen sulfide is in the form of hydrosulfide ion (HS–), which does not have an odor. However, at pH below nine, hydrogen sulfide is released from the wastewater [10]. In addition, chemical reagents used at WWTP for treatment may be odorous and can produce odors during the treatment process. Common emissions from WWTP include hydrogen sulfide, mercaptans, VOC, ammonia and some nitrogen containing compounds.
At WWTPs, compounds are only noticeably odorous if the compound is volatilized into the air. For example, hydrogen sulfide dissolved into wastewater has little odor. However, turbulence in the wastewater, increased temperature, solubility changes and pH changes can all cause compounds, such as hydrogen sulfide, to volatilize to the gas phase, thereby producing a higher level of odor. In addition, the surrounding community often considers the odors coming from WWTPs as being problematic. The WWTPs have to evaluate the source of the odors. Many studies have determined the main sources of odors at WWTPs to be the primary settlers, sludge-digestions tanks, sludge thickening, dewatering areas, raw wastewater, centrifugation and disposal systems [ 11]. Overall, two of the main contributors to odors are the primary treatments and sludge-handling areas. At this point, WWTPs have to consider what type of odor control to use.
Odor remediation methods
WWTPs can use a variety of methods to remove odors. The main odor control categories into which these methods fall are the following: operation control methods, liquid-phase addition and vapor-phase methods (Figure 2).

Figure 2. Odor-removal methods, such as the vapor-line system shown here, involve treating the air that contains the odor-causing species
Operation control methods. Operation control methods involve changing a process or operation in order to reduce the odors. Operators can help prevent offensive odors by maintaining the correct dissolved oxygen concentration, for example, or by preventing excess sludge from degrading, preventing overloading and many other simple maintenance techniques. However, operators are often limited in what changes they can make and may lack the resources to make the necessary changes. If operators are unable to make changes to these processes, or the changes do not fix the odor problem, then they may look to other methods of odor control.
Liquid-phase addition. Liquid-phase addition involves adding a chemical to the wastewater in order to control the odors. The chemical addition may either prevent odor-causing chemicals from forming or may react with the odorous compounds to produce a safer, non-odor-causing species. Engineers may be able to take advantage of other benefits with this technique; namely, corrosion control. For instance, hydrogen sulfide will react with many of the liquid-phase chemicals, resulting in hydrogen sulfide not being released into the air stream. Therefore, hydrogen sulfide will not corrode the WWTP equipment.
Liquid-phase chemicals include, but are not limited to, oxidizers, pH modifiers and bactericides. Oxidizers typically react with hydrogen sulfide and other sulfur compounds. Bactericides work by either inactivating or killing anaerobic bacteria. The pH modifiers tend to prevent the hydrogen sulfide from volatizing into the air. The most common chemicals added to the wastewater are iron salts, ozone, nitrates, chlorine and hydrogen peroxide.
Vapor-phase approaches
Vapor-phase methods involve the treatment of the air or vapor that contains the odorous compounds. There are many different vapor-phase technologies that WWTPs utilize, including, but not limited to, scrubbers, biofiltration, activated carbon, masking agents and natural odor control using plant oils as the active ingredients to neutralize the odors. Below is a brief summary of the basic way each of these methods works.
Wet scrubbers. Wet scrubbers pump the contaminated air into an aqueous solution before it goes to the ambient air. The odorous compounds go into the liquid, and then the odor-causing chemicals react with the solution. Wet scrubbers typically use sodium hypochlorite, potassium permanganate, hydrogen peroxide or sodium hydroxide in the aqueous solution. If hydrogen sulfide is the main odor concern, sodium hydroxide is often used. However, if mercaptans or ammonia are also present, then a multi-stage scrubber is used, with both sodium hydroxide and sodium hypochlorite utilized in separate stages.
Biofiltration. Biofiltration uses soil, compost or some other material, such as a substrate for bacteriological population. The microbes remove odors from the air through the media. In order for the microbes to interact with the odorous compounds, there must be a large residence time in the media. By lowering the velocity of the air going through the media, longer residence times result. Biofiltration works on odors that are both biodegradable and water-soluble, including hydrogen sulfide and other sulfur-containing compounds. They do not work effectively on chemicals containing nitrogen.
Activated carbon. Activated carbon works by having the contaminated air stream pass through this adsorbent material. Activated carbon is porous, with a large surface area, which allows it to adsorb the odorous chemicals in the air stream. While activated carbon works well on sulfur-containing compounds, it is not as effective at treating nitrogen-based compounds [12].
Masking agents. Masking agents are chemicals that are sprayed into the air to cover up the odors produced at the WWTP. Masking agents use fragrances to cover up the odors. They often work by adhering to the outside of the odor molecule. After a short period, the fragrance and the odor separate, leaving the odor behind. While masking agents give an immediate cover to the odor, the odor will eventually return. As a result, not everyone in nearby communities will be happy with the overall outcome.
Natural solutions. Finally, natural odor control solutions can fully neutralize odors, and are atomized or vaporized into the air. These odor-control products contain natural plant oils, food-grade surfactant and water, and are both safe for the environment and the local community. When the natural odor control product is atomized into the air, small droplets are produced that cover a large surface area and attract odorous gas molecules through electrostatic charges. The electrostatic charge also facilitates the attraction of malodor molecules to the droplet surface whereby they absorb into the droplet. Once the malodor is in the droplet, odor neutralization occurs. The malodor then either naturally biodegrades in the droplet or an acid/base reaction takes place, producing a non-hazardous organic-salt and water.
Natural odor-control solutions often use plant oils. These products have the capability of neutralizing a broad spectrum of odorous chemicals, including hydrogen sulfide, mercaptans, VOCs, ammonia and amines. These solutions can be atomized into the air at WWTPs via air atomization systems, hollow bladed fans or by vapor-phase systems. The air atomization systems use water and nozzles to atomize the product into the air. The nozzle system can be suited to most applications at a WWTP. The hollow-bladed fan system is a uniquely designed system that dispenses the product into the open air.
Current vapor-phase systems offer a simple, effective and economical delivery system specifically developed to disperse the water-based odor-eliminating neutralizers. Vapor-phase units are designed to produce sub-micron droplets of products. This is accomplished by a centrifugal pressure blower that intakes atmospheric air. The intake air stream is sprayed with product, pulled through the blower and finally dispersed on the outlet side of the blower. Additional water is not required. The sub-micron size droplet allows for a more effective dry vapor that absorbs more odor molecules than atomization systems.
In recent years, WWTPs have come under an increasing level of scrutiny from their respective surrounding communities. The perception of odors coming from WWTPs has resulted in many complaints from the community. Investigating the various odor-control methods described in this article can help minimize complaints. Overall, the best solution would be one that removes a broad spectrum of odors, and is safe for both the community and the environment.
Edited by Scott Jenkins
References
1. U.S. Environmental Protection Agency, “Reference Guide to Odor Thresholds for Hazardous Air Pollutants Listed in the Clean Air Act Amendment of 1990.” https://nepis.epa.gov/Exe/ZyPDF.cgi/2000BHG5.PDF?Dockey=2000BHG5.PDF
2. Mayo Clinic, “Diseases and Conditions Hyponatremia.” http://www.mayoclinic.org/diseases-conditions/hyponatremia/basics/definition/con-20031445
3. The National Institute for Occupational Safety and Health (NIOSH), “Ammonia (Anhydrous),” https://www.cdc.gov/niosh/ipcsneng/neng0414.html.
4. Leonardos, G., Kendall, D., Barnard, N., “Odor Threshold Determinations of 53 Odorant Chemicals,” http://dx.doi.org/10.1080/00022470.1969.10466465.
5. U.S. Occupational Safety and Health Administration, “Hydrogen Sulfide” Definition, https://www.osha.gov/SLTC/hydrogensulfide/hazards.html.
6. U.S. Centers for Disease Control and Prevention, “Occupational Health Guidelines for Hydrogen Sulfide,” https://www.cdc.gov/niosh/docs/81-123/pdfs/0337.pdf.
7. National Academy of Science, “Hydrogen Sulfide Acute Exposure Guideline Levels,” https://www.ncbi.nlm.nih.gov/books/NBK208170/.
8. New Jersey Department of Health and Human Services, “Hazardous Substance Factsheet: Ethyl Mercaptan,” nj.gov/health.
9. U.S. EPA, “Toluene – Hazard Summary,” www.epa.gov/sites/production/files/2016-09/documents/toluene.pdf.
10. Lebrero, R., Bouchy, L., Stuetz, R. and Muñoz, R., “Odor Assessment and Management in Wastewater Treatment Plants: A Review,” http://www.tandfonline.com/doi/abs/10.1080/10643380903300000.
11. U.S. EPA, “Detection, Control and Correction of Hydrogen Sulfide Corrosion in Existing Wastewater Systems,” https://nepis.epa.gov/Exe/ZyPDF.cgi/200045MK.PDF?Dockey=200045MK.PDF.
12. Zhang, L., DeSchryver, P., DeGusseme, DeMuynck, W., Boon, N. and Verstaete, W., “Chemical and Biological Technologies For Hydrogen Sulfide Emission Control In Sewer Systems,” http://www.sciencedirect.com/science/article/pii/S0043135407004745.
Author
 Laura Haupert is the director of research and development for OMI Industries (1300 Barbour Way, Rising Sun, IN 47040; Phone: 1-812-438-9218; Email: lhaupert@omi-industries.com; Website: odormanagement.com). At OMI, Haupert helps develop natural solutions for the treatment of industrial odors, and supports the company’s Ecosorb technology, which is designed to eliminate odors without hazardous chemicals. Haupert earned her B.S. in chemistry (biochemistry concentration) from Manchester College and holds a Ph.D. in physical chemistry from Purdue University, where she worked in the area of solvated clusters. Haupert also completed post-doctoral research at Purdue.
Laura Haupert is the director of research and development for OMI Industries (1300 Barbour Way, Rising Sun, IN 47040; Phone: 1-812-438-9218; Email: lhaupert@omi-industries.com; Website: odormanagement.com). At OMI, Haupert helps develop natural solutions for the treatment of industrial odors, and supports the company’s Ecosorb technology, which is designed to eliminate odors without hazardous chemicals. Haupert earned her B.S. in chemistry (biochemistry concentration) from Manchester College and holds a Ph.D. in physical chemistry from Purdue University, where she worked in the area of solvated clusters. Haupert also completed post-doctoral research at Purdue.