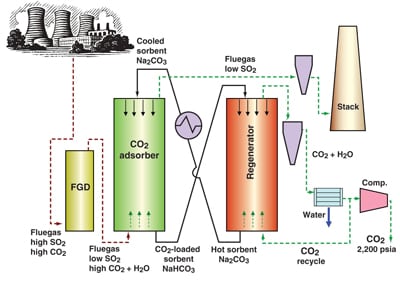
 While monoethanolamine (MEA) is the traditional solvent for scrubbing CO2 from fluegas, more effective methods, including solid absorbents, are being developed (see CE, December 2008, pp. 16–20). Sodium carbonate, for example, is the sorbent in a process being developed, with DOE support, by Research Triangle Institute (RTI, Research Triangle Park, N.C.; www.rti.org). Desulfurized fluegas from a wet scrubber is passed through a fluidized bed of Na2CO3 particles, which adsorb CO2 to form bicarbonate at 50–80°C. When the particles are loaded they are heated with steam or CO2 to about 120°C to drive off a 99%-pure CO2 stream and regenerate Na2CO3 (diagram).
While monoethanolamine (MEA) is the traditional solvent for scrubbing CO2 from fluegas, more effective methods, including solid absorbents, are being developed (see CE, December 2008, pp. 16–20). Sodium carbonate, for example, is the sorbent in a process being developed, with DOE support, by Research Triangle Institute (RTI, Research Triangle Park, N.C.; www.rti.org). Desulfurized fluegas from a wet scrubber is passed through a fluidized bed of Na2CO3 particles, which adsorb CO2 to form bicarbonate at 50–80°C. When the particles are loaded they are heated with steam or CO2 to about 120°C to drive off a 99%-pure CO2 stream and regenerate Na2CO3 (diagram).
The process "uses only about half the energy of amine processes and you don’t have to handle any corrosive liquid," says Raghubir Gupta, a senior scientist.
RTI has tested the process at a scale of about 1/3-m.t./d of CO2 and is now building a 10-m.t./d pilot unit.
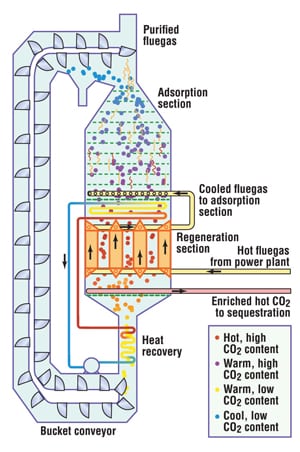
… and another with adsorbing beads
In a concept being developed by Adsorption Research, Inc. (ARI; Dublin, Ohio; www.adsorption.com), adsorbent beads are delivered to the top of an adsorber column by a bucket elevator and fall down through an adsorption section, counter to upflowing, cooled fluegas. The beads’ passage is slowed by a series of perforated trays to provide a residence time of about 1-1/2 min for carbon dioxide adsorption.
Next, the beads drop through a heat-exchanger section, where they are indirectly heated to 400 – 600°F by incoming fluegas. The heat releases the CO2, which is withdrawn through a perforated pipe. Simultaneously, the gas is cooled to about 125°F for the adsorption step.
In laboratory tests, using a zeolite or a proprietary adsorbent, the process has achieved 89% CO2 recovery, with a purity of 99%, says Kent Knaebel, president of ARI. He adds that, unlike MEA adsorption, the process requires little parasitic heat or cooling energy and the adsorbent is not degraded by SO2, NOx or O2. He estimates that a commercial unit could process 15,000 tons/d of CO2 from a 500-MW power plant for under $20/ton, compared with about $40/ton for MEA.