This column is based on “Isopropyl Alcohol Production from Acetone – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/isopropyl-alcohol-production-cost.
Isopropyl alcohol (isopropanol or 2-propanol) is a colorless and flammable liquid with a strong odor. Isopropyl alcohol was among the first petrochemical products to be industrially manufactured — it has been produced since 1920. At that time, isopropanol was generated through the indirect hydration of propylene, a process that uses sulfuric acid to react with propylene and generate a compound that will react with water to produce isopropanol. Today, isopropanol is one of the most produced C1 to C5 alcohols, ranking third in commercial production, behind methanol and ethanol.
The main industrial applications of isopropyl alcohol are as a chemical intermediate and as a solvent in the manufacturing of cements, primers, paints, varnishes, skin cleaners, perfumes, lotions, shampoos and deodorants. It is also used as a disinfectant and antiseptic.
The process
The present analysis discusses an industrial process for isopropanol production. The process under analysis comprises two main sections: (1) reaction; and (2) purification (Figure 1).
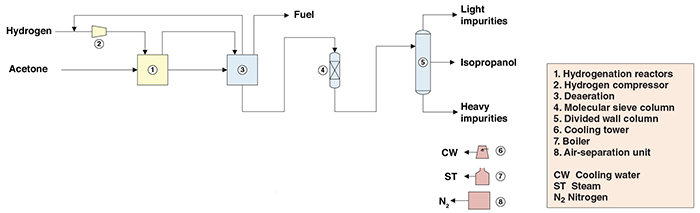
Figure 1. The diagram shows a process for isopropanol production via acetone hydrogenation
Reaction. Hydrogen is compressed and fed, along with liquid acetone, to the upper part of a circulation reactor. The circulating mixture passes through a gas-liquid separation vessel. Part of the liquid is cooled — removing the heat generated by the exothermic reaction — and recycled to the reactor. The remainder is cooled and routed to a second reactor, followed by a second vapor-liquid separation stage. The liquid portion obtained after the second vapor-liquid separation is sent to the purification stage. The vapor portion is mixed with the vapors from the first gas-liquid separation and also sent to purification.
Purification. In the purification stage, the vapors from the reaction are fed to a condenser, where isopropanol and acetone are recovered. Most of the uncondensed vapors are recycled to the hydrogen compressors. The remaining portion of the hydrogen-rich gaseous stream, the condensate from the condenser and the liquid product from the reaction are fed to a deaeration tank to remove any gases dissolved in the crude liquid product. The crude product is then subjected to dehydration by means of molecular sieves, and finally fed into a purification column, from which purified isopropanol product is withdrawn as a side stream. Low-boiling components are separated as the column’s overheads product, while high-boiling impurities are obtained as the bottoms product.
Production pathways
The main raw materials for isopropanol manufacture are propylene and acetone. Propylene is reacted with water directly, or indirectly (in the presence of sulfuric acid), to form isopropanol. Acetone, in turn, passes though a hydrogenation process. Figure 2 presents different pathways for isopropanol production.
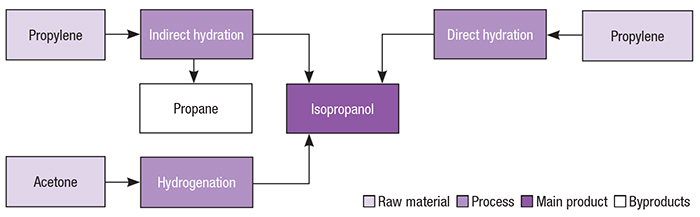
Figure 2. Isopropanol production can occur via different pathways, as shown here
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce isopropanol was about $1,000 per ton of isopropanol in the fourth quarter of 2016. The analysis was based on a plant constructed in the U.S. with capacity to produce 60,000 metric ton per year of isopropyl alcohol.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.