Electrochemical water-treatment technologies facilitate the adsorption of ions, effectively removing contaminants such as calcium, magnesium, bromine and more. Such processes, however, are often hampered by the high costs associated with electrode regeneration and the use of ion-exchange membranes. A new electrochemical system developed by researchers from Samsung Electronics Ltd. (www.samsung.com) and Sungkyunkwan University (both Seoul, South Korea; www.skku.edu) takes advantage of the electrode-regeneration cycle to harvest additional power, while eliminating ion-exchange membranes altogether.
Source: Samsung
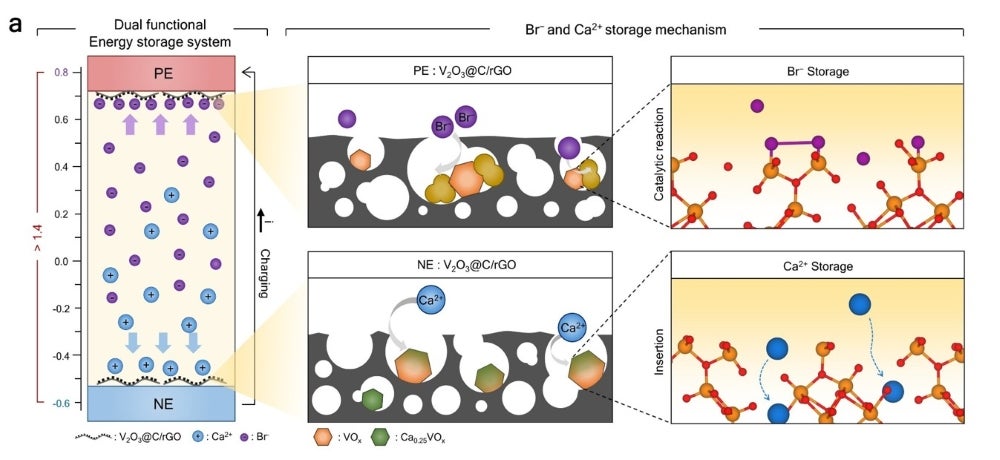
At the center of this symmetric redox-active system is an electrode composed of vanadium oxide/carbon hybrids grown on reduced graphene oxide, which can “store and release both divalent cations and halide anions in a Faradaic manner.” This configuration enables a reported 200% increase in ion storage capacity and a 20% increase in storage rate. Rather than relying on electrostatic forces to move ions, as with conventional electrodes, this new electrode supports spontaneous, direct electron exchange, eliminating the need for an ion-exchange membrane. Additionally, the symmetrical nature of the module also simplifies electrode fabrication procedures.
Existing electrode technologies used for water treatment need periodic regeneration, which requires an external power supply and can lead to detrimental ion leakage that can prevent the utilization of stored energy. The new electrode developed by Samsung and SKKU can reportedly be regenerated spontaneously without the supply of additional power. Crucially, the electrode can recover stored energy and provide power to other devices, all while consuming about half the energy as other electrochemical water-treatment systems. The ability to recover and store energy within the water-treatment module makes it suitable as a backup power device. According to the research team, such a system with “efficient ion-storage and release technology from wastewater” has not been explored previously.
The researchers have demonstrated the module over a variety of wastewater concentrations, from high-concentration industrial wastewater to less concentrated household streams, highlighting its selective ion capture and fast ion storage and release kinetics. This work was detailed in a recent issue of Joule.