Ammonia manufacturing with carbon capture (blue ammonia) has emerged as the most practical near-term option for deep decarbonization of ammonia production. Evaluated here are eight technology configurations for decarbonization strategies with blue ammonia production
Ammonia is increasingly viewed as an essential contributor to low-carbon energy systems, serving as a carrier of hydrogen, a carbon-free fuel and a vital chemical feedstock. Markets for green ammonia (NH3 made using hydrogen from the renewable-energy-powered electrolysis of water) remain limited. However, blue ammonia — referring to ammonia production with associated capture of carbon dioxide emissions — offers a practical approach for the near-term, large-scale deployment of deep decarbonization for ammonia production. Although global demand for low-carbon ammonia is still developing, deep decarbonized blue ammonia is emerging as the most practical pathway. Production of blue ammonia is supported by established reforming technologies, expanding carbon capture projects and a favorable natural gas supply.
This article evaluates eight process configurations for a 3,500 metric ton per day (m.t./d) ammonia plant on the U.S. Gulf Coast. This region offers a host of enabling factors that allow deep decarbonization of ammonia production at scale, including access to low-cost natural gas, significant carbon-dioxide storage capacity and mature industrial infrastructure. Together, these factors allow deep decarbonization at a commercially viable scale, positioning Gulf Coast blue ammonia as a bridge technology toward longer-term hydrogen-economy goals.
The eight technology configurations compared here include autothermal reforming, partial oxidation, convective reforming, nitrogen wash, two-stage pressure-swing adsorption for hydrogen purification and hydrogen-rich firing. All schemes include feed purification, pre-reforming (where applicable) and integrated steam systems. Scope 1 and Scope 2 carbon intensities are estimated using standardized capture efficiency and regional grid assumptions. Results highlight differences in carbon capture performance and natural gas consumption. The article provides a framework for technology selection that balances emissions reduction, process integration and indicative economics.
Technology configurations
 The following blue ammonia production process schemes were broadly evaluated for high to deep-decarbonization options:
The following blue ammonia production process schemes were broadly evaluated for high to deep-decarbonization options:
• Case 1: SMR + SR + PCCS
• Case 2: SMR + SR + PoPCCS
• Case 3: ATR + NWU + PCCS
• Case 4: ATR + NWUR + H2RFire + PCCS
• Case 5: ATR + PSA⁺ + H2Rfire + PCCS
• Case 6: ATR + CR + PSA⁺ + PCCS
• Case 7: POx + PSA⁺ + PCCS
• Case 8: POx + NWU + PCCS
A brief review of catalytic methane pyrolysis (CMP) is also included as an emerging low-carbon alternative.
All cases were evaluated under a common basis. Each simulation estimated utilities, carbon capture performance, and Scope 1 and Scope 2 emissions for a 3,500 m.t./d ammonia plant with carbon dioxide compressed to 155 barg for sequestration. Post-combustion capture was set at 95% (maximum), while pre-combustion capture exceeded 99.99%. Scope 1 emissions reflect direct plant releases, and Scope 2 emissions reflect grid power imports for the ASU and major outside battery limits (OSBL) units.
Hydrogen purification options were examined by comparing nitrogen wash and two-stage PSA, with and without recycle, to understand their impact on recovery, energy use and integration.
Case descriptions
Each configuration is briefly described below to highlight the major process steps, integration features, and carbon management approach used in the evaluation. All cases are illustrated in the accompanying process schematics (Figures 1–8).
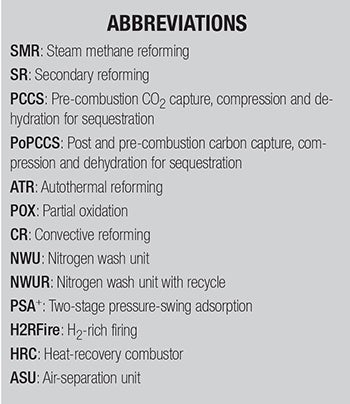
Case 1: SMR with SR and pre-combustion capture. This base case (Figure 1) represents a conventional but modern configuration widely deployed in commercial ammonia plants. It includes steam methane reforming followed by secondary reforming, along with compression and dehydration of captured pre-combustion carbon dioxide for sequestration. This configuration provides moderate decarbonization with well-established technology and operational reliability.
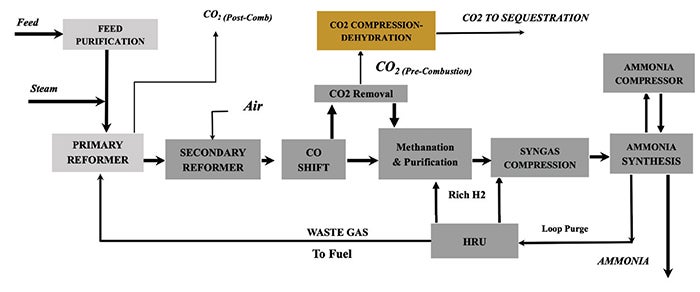
FIGURE 1. The base case represents a conventional, but modern, configuration widely deployed in commercial ammonia plants
Case 2: SMR with SR and combined pre-combustion and post-combustion capture. This case (Figure 2) builds on Case 1 by capturing carbon dioxide from the reformer stack and combining it with the pre-combustion carbon dioxide stream for compression and dehydration, enabling deeper decarbonization. This approach allows for deeper decarbonization while maintaining the core SMR/SR process configuration.
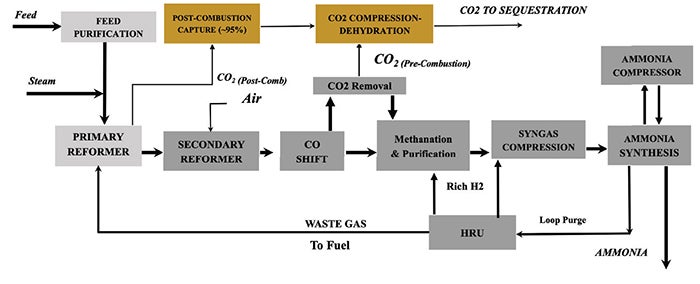
FIGURE 2. This case builds on Case 1 by capturing carbon dioxide from the reformer stack and combining it with the pre-combustion carbon dioxide stream
Case 3: ATR with nitrogen wash and pre-combustion capture. This configuration (Figure 3) employs autothermal reforming (ATR) operated at the lowest commercially viable steam-to-carbon ratio. Oxygen and process nitrogen are supplied by an integrated air separation unit (ASU). Hydrogen purification is performed in a nitrogen wash unit (NWU). Separate fired heaters provide process preheating and high-pressure steam superheating, using natural gas blended with plant off-gas as fuel.
FIGURE 3. This configuration employs autothermal reforming (ATR) operated at the lowest commercially viable steam-to-carbon ratio
Case 4: ATR with nitrogen wash recycle, hydrogen-rich firing, and pre-combustion capture. This case (Figure 4) extends Case 3 by recycling carbon-containing streams to the ATR and using internally generated hydrogen-rich fuel for fired heaters. Recycling increases front-end equipment size and oxygen demand but significantly reduces carbon intensity, potentially improving project value under carbon-pricing mechanisms or low-carbon certification requirements.
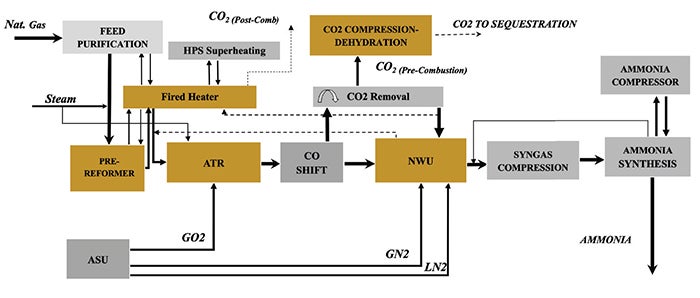
FIGURE 4. This case extends Case 3 by recycling carbon-containing streams to the ATR and using internally generated hydrogen-rich fuel for fired heaters
Case 5: ATR with two-stage PSA, hydrogen-rich firing, and pre-combustion capture. This scheme (Figure 5) replaces the nitrogen wash unit with a two-stage pressure-swing adsorption (PSA+) system to achieve hydrogen recovery comparable to that of nitrogen wash. Fired heaters utilize hydrogen-rich fuel for deep decarbonization. As in Case 4, the front end enlarges due to higher hydrogen production requirements, but lower carbon intensity may offset increased capital costs depending on regional carbon policy and project financing terms.
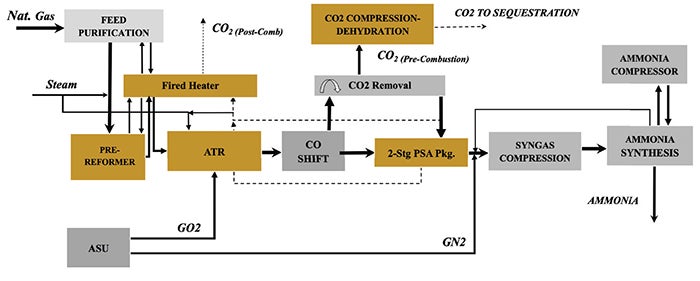
FIGURE 5. This scheme replaces the nitrogen wash unit with a two-stage pressure-swing adsorption (PSA+) system to achieve hydrogen recovery comparable to that of nitrogen wash
Case 6: ATR with convective reforming, PSA+ and pre-combustion capture. This case (Figure 6) adds a convective reformer in series with the ATR and operates at a higher steam-to-carbon ratio with PSA+. The convective reformer recovers high-temperature heat to provide additional reforming duty, reducing natural gas and oxygen demand, but lowering high-pressure steam generation. The larger front end is balanced by improved carbon intensity under deep decarbonization targets.
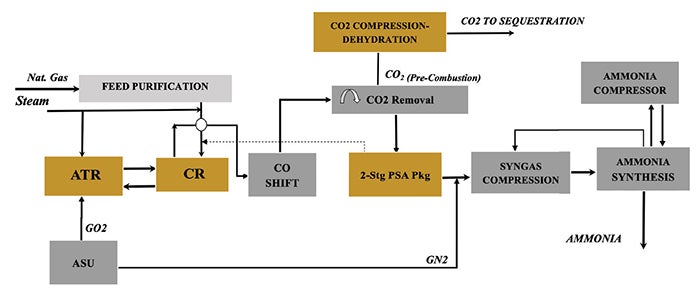
FIGURE 6. This case adds a convective reformer in series with the ATR and operates at a higher steam-to-carbon ratio
Case 7: POx with PSA+ and pre-combustion capture. This scheme (Figure 7) uses partial oxidation (POx) and PSA+; the POx operates at a much higher temperature than ATR and requires no catalyst. Oxygen demand is slightly higher, but methane slip is significantly lower. Integrated heat recovery for process preheating and high-pressure steam superheating eliminates the need for direct-fired heaters, further reducing emissions.
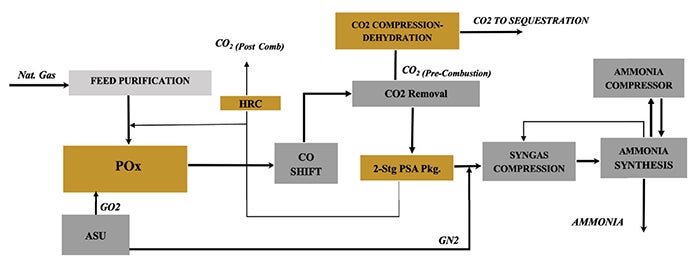
FIGURE 7. This scheme uses partial oxidation (POx) and PSA+; the POx operates at a much higher temperature than ATR and requires no catalyst
Case 8: POx with nitrogen wash and pre-combustion capture. This case (Figure 8) is similar to Case 7, but employs a nitrogen wash unit for hydrogen purification. No nitrogen wash recycle is applied. Despite the absence of recycling, the configuration still achieves approximately 97% carbon capture due to the inherently low methane slip characteristic of partial oxidation technology.

FIGURE 8. This case is similar to Case 7, but employs a nitrogen wash unit for hydrogen purification
Steam system
The high-pressure steam level and degree of superheat were appropriately selected for each configuration to minimize fired-heater duty and reduce overall carbon intensity. Steam turbine drives were employed for major compression equipment (synthesis gas and ammonia compressors) when sufficient high-pressure (HP) or medium-pressure (MP) superheated steam was available. Otherwise, motor drives were used to maintain energy efficiency. Surplus superheated high-pressure steam was expanded through back-pressure or condensing turbines for power generation, with net electricity credited as plant export.
All other OSBL equipment, including the ASU, used motor drives, while the high-pressure boiler feedwater pump operated on steam or on motor as required. Process steam for the water-gas shift reactors was supplied from the process condensate stripper and the medium-pressure steam header. A minimum steam-to-gas ratio of 0.5 was maintained at the shift reactor inlet, with higher ratios applied as needed to control shift outlet temperature within catalyst and metallurgical limits.
Emissions and carbon intensity
Carbon intensity calculations encompass Scope 1 direct emissions (combustion and process releases) and Scope 2 emissions from imported grid electricity (Louisiana regional grid factors were considered here). Pre-combustion capture efficiency was set at >99.99% (conventional acid gas removal), while post-combustion capture was limited to 95% (current amine technology). Upstream natural gas emissions are excluded, consistent with project-level carbon accounting boundaries.
Figure 9 illustrates significant differences across configurations. Case 2 achieves modest improvement through combined capture but remains constrained by the 95% post-combustion ceiling, leaving residual flue gas emissions that prevent attainment of the ultra-low carbon-intensity thresholds required for future certification schemes.
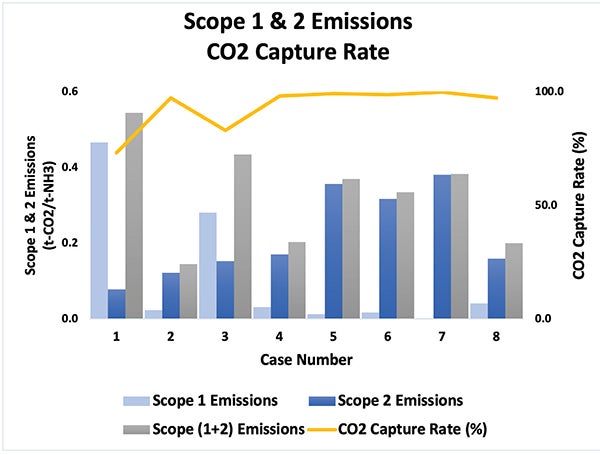
FIGURE 9. Emissions differences among the different cases are shown here
Cases 4–6 deliver the lowest carbon intensities by concentrating CO₂ in syngas for efficient pre-combustion capture, maximizing hydrogen recovery, employing carbon recycling or convective reforming, and utilizing hydrogen-rich fuel that eliminates fired-heater stack emissions — enabling capture rates exceeding 98%.
Cases 7–8 achieve comparable performance due to inherently low methane slip, stable high-temperature conditions that favor complete conversion, and the elimination of direct-fired heaters through integrated heat recovery. Multiple technology pathways achieve similar targets, offering developers flexibility based on site-specific conditions and risk preferences.
Hydrogen purification variations
ATR and POx with PSA differ from the nitrogen wash in the separation method. PSA uses cyclic adsorption to remove impurities, while nitrogen wash relies on cryogenic separation integrated into the synthesis loop. This difference influences energy use, integration and flowsheet optimization. Single-stage PSA provides lower hydrogen recovery, increasing feed demand, but its larger off-gas stream supports steam generation and reduces net power consumption. Two-stage PSA+ achieves recoveries comparable to those of nitrogen wash, though at the cost of additional compression duty.
Nitrogen wash offers high hydrogen recovery and direct loop integration, suited for large plants, but requires refrigeration, is more capital-intensive and offers less modular flexibility. The choice depends on site-specific economics, integration potential and operating constraints.
Comparison of feedstock, fuel and primary utility needs provides insight into operating characteristics across the eight cases. Figure 10 shows apparent differences in feed, fuel and oxygen use. Case 2 uses moderate natural gas without oxygen, but lowering carbon intensity through hydrogen-rich firing would increase natural gas demand and require significant modifications to the heaters.
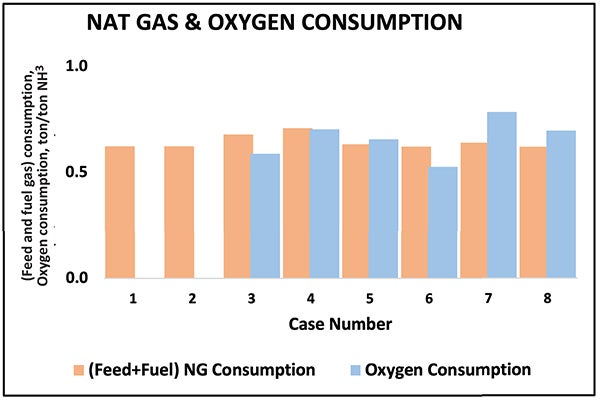
FIGURE 10. The graph shows the apparent differences in feed, fuel and oxygen use among the different cases
ATR-based Cases 4 and 5 consume more natural gas and oxygen because recycling and higher syngas flow generate hydrogen-rich fuel. Case 6 behaves differently: the convective reformer recovers high-temperature heat for additional reforming, reducing feedstock and oxygen consumption but lowering high-pressure steam generation. Consequently, the steam system cannot support large syngas and ammonia compressors, requiring motor drives.
POx-based Cases 7 and 8 achieve deep decarbonization through simplified heat integration: the availability of additional process heat enables process preheating and steam superheating, eliminating the need for fired heaters while maintaining exceptionally low methane slip and stable carbon-capture performance.
Process recycle reduction
Lower carbon intensity can be achieved by minimizing recycle, which requires reducing CH4 and CO in the syngas/H₂ stream. Methane slip is primarily addressed under reforming operating conditions set for each configuration, leaving limited scope for further reduction. CO, however, can be lowered to single‑digit ppm levels using either conventional methanation or selective catalytic oxidation.
Residual CO removal
Selective CO oxidation represents a future improvement option for thoroughly removing residual CO from shifted syngas (down to single-digit ppm levels) to minimize off-gas recycle to the front end (ATR or POx), reduce carbon intensity and lower both capital and operating costs.
Selective CO oxidation removes CO without a hydrogen penalty or inert buildup, improving loop efficiency and carbon dioxide capture. Challenges include sensitivity to gas impurities, limited commercial experience and the high cost of precious-metal catalysts. It offers promising reductions in carbon intensity for large-scale ammonia projects, but long-term reliability and economics require careful evaluation before widespread adoption.
Methanation is not a viable option for deep decarbonization schemes because it consumes hydrogen and forms methane, which dilutes the synthesis loop, increases purge losses and raises carbon intensity by converting captured carbon into an uncaptured form.
Techno-economic viability
Economic performance review highlights essential cost and long-term value differences across the eight cases. Figure 11 shows that Case 2 (SMR with SR and post-combustion capture) yields the lowest capital and operating costs among deep decarbonization pathways. Still, this advantage weakens when operational reliability is considered. At 3,500 m.t./d. scale, the post-combustion system becomes extremely large, creating challenges with solvent handling, corrosion, foaming, aerosol formation and parasitic power demand — reducing availability and increasing lifetime costs. Case 2 is also constrained by the 95% post-combustion capture ceiling (Figure 9), preventing the ultra-low carbon intensity required for future certification.
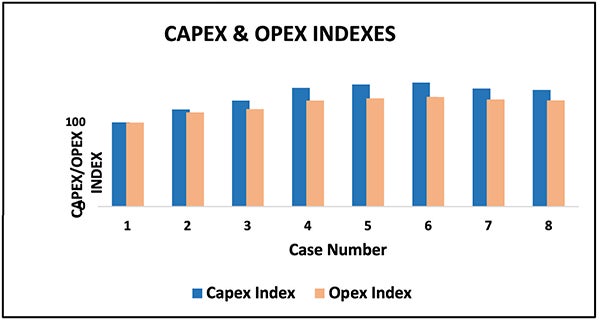
FIGURE 11. This graph shows that Case 2 (SMR with SR and post-combustion capture) yields the lowest capital and operating costs among the deep-decarbonization pathways
Hydrogen-rich firing has been considered to mitigate furnace emissions in Case 2, but application in large SMR heaters requires significant design modifications. In contrast, ATR and POx configurations (Cases 4–8) offer higher reliability, simpler carbon capture, better scalability and more substantial long-term economic value, despite higher initial capital costs.
Emerging low-C pathways
Catalytic methane pyrolysis (CMP) offers a promising route to very low-carbon ammonia by generating solid carbon rather than carbon dioxide when converting methane to hydrogen. This would eliminate CO2-capture and storage requirements. Although at the pilot scale, integration of pyrolytic hydrogen into ammonia synthesis could lower carbon intensity in the near term.
CMP converts methane into solid carbon and hydrogen, with no process-related carbon dioxide emissions. Each ton of ammonia produced yields approximately 0.54 tons of solid carbon, which is potentially saleable into carbon-black markets, if quality permits. However, with global carbon black capacity near 15 million tons annually and slow demand growth, pyrolysis can support only limited ammonia production before market saturation.
CMP economics depend strongly on carbon black revenue; wider adoption requires new solid carbon applications. Key technical challenges include catalyst life, reactor performance and stable high-temperature operation. The absence of process carbon dioxide makes pyrolysis attractive where carbon capture faces geographic or policy barriers.
Other emerging options include electrified reforming, which may complement ATR and POx designs as power grids decarbonize.
Concluding remarks
This study shows that deep decarbonization of ammonia production is driven by how carbon is managed within the process, not by the lowest front-end capital cost.
Case 2 appears economically attractive, but its dependence on post-combustion capture from large SMR furnaces creates a fundamental process and regulatory constraint. Flue-gas capture is limited to roughly 95% removal, leaving unavoidable residual emissions that prevent compliance with the 98–99% capture levels now emerging in low-carbon ammonia certification schemes. At a 3,500-ton/d scale, the amine system becomes exceptionally large, increasing solvent degradation, corrosion, aerosol formation and parasitic energy use, which together reduce availability and inflate lifetime cost. Converting SMR furnaces to hydrogen-rich firing would require a major redesign of the heaters and still cannot eliminate the intrinsic disadvantages of dilute flue-gas CO2 capture. These factors undermine the bankability of Case 2, despite its lower nominal cost.
ATR-based (Cases 4–6) and POx-based (Cases 7–8) configurations provide a fundamentally stronger decarbonization pathway. Both concentrate carbon in high-pressure syngas, enabling greater than 98% capture through conventional acid-gas removal, while eliminating large flue-gas treatment systems. Integrated heat recovery and hydrogen-rich firing further suppress residual emissions and improve operating stability. These attributes align more closely with CO2 transport, sequestration and certification requirements, making ATR and POx the preferred platforms for projects that have reached the final investment decision.
Catalytic methane-pyrolysis and electrified-reforming technologies offer long-term promise, but remain at an early stage of commercial readiness. Ultimately, technology selection must balance cost, reliability, carbon intensity and long-term regulatory resilience.
Edited by Scott Jenkins
Acknowledgement
All diagrams and graphs appearing in the article were provided by the author.
Reference
1. Arora, V.K., Enabling Clean Ammonia: Practical Lessons from a Full Plant Conversion, Chem. Eng., Dec. 2025, pp. 34–37.
Author
 V.K. Arora, P.E., leads Kinetics Process Improvements Inc. (KPI; 16000 Park Ten Place, Suite 903, Houston, TX 77084; Email: vka@kpieng.com; Phone: 281-717-1629; Website: kpieng.net). Arora is a chemical engineer with over 35 years of experience delivering value-driven, practical, and economical process solutions across petrochemical, refining, and syngas facilities. A Texas-licensed professional engineer and IIT Delhi graduate, he has guided KPI for more than 20 years, specializing in high-return brownfield revamps, debottlenecking, and techno-economic feasibility studies. His work spans ethylene plants, propane dehydrogenation, acrylic acid and esters, ammonia and methanol facilities, and integrated low-carbon process solutions. He holds four ammonia process patents and has held senior leadership roles at the following companies: Lummus Technology, KBR Inc., Saudi Basic Industries Corp.(SABIC), Reliance Industries and Technip.
V.K. Arora, P.E., leads Kinetics Process Improvements Inc. (KPI; 16000 Park Ten Place, Suite 903, Houston, TX 77084; Email: vka@kpieng.com; Phone: 281-717-1629; Website: kpieng.net). Arora is a chemical engineer with over 35 years of experience delivering value-driven, practical, and economical process solutions across petrochemical, refining, and syngas facilities. A Texas-licensed professional engineer and IIT Delhi graduate, he has guided KPI for more than 20 years, specializing in high-return brownfield revamps, debottlenecking, and techno-economic feasibility studies. His work spans ethylene plants, propane dehydrogenation, acrylic acid and esters, ammonia and methanol facilities, and integrated low-carbon process solutions. He holds four ammonia process patents and has held senior leadership roles at the following companies: Lummus Technology, KBR Inc., Saudi Basic Industries Corp.(SABIC), Reliance Industries and Technip.